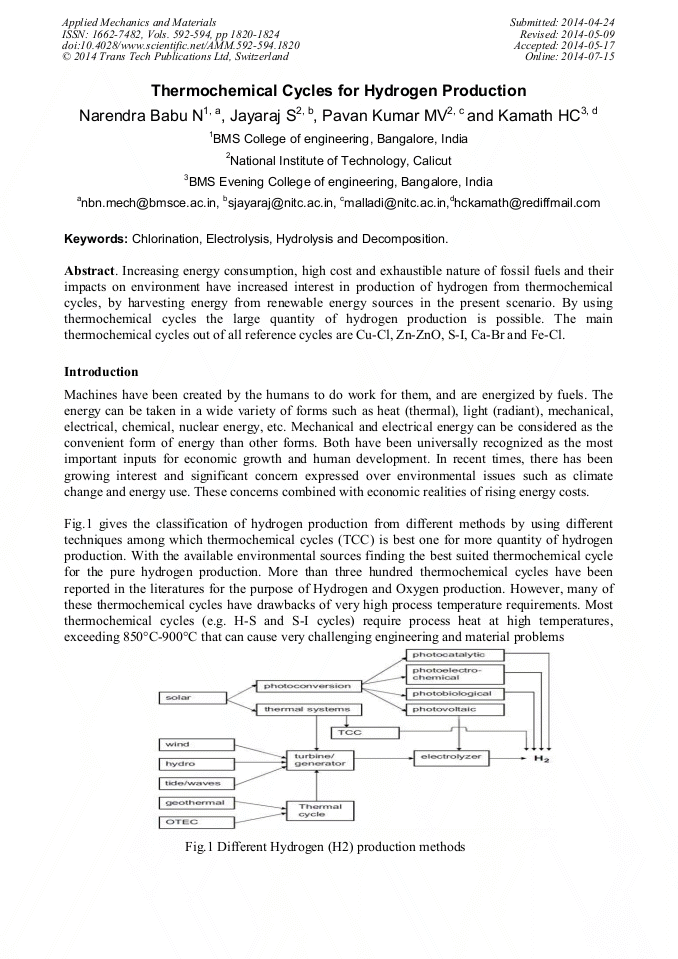
[1]
Abanades S, Charvin P, Flamant G, Neveu P: Screening of water-splitting thermochemical cycles potentially attractive for hydrogen production by concentrated solar energy. Energy 2006; 31: 2805-22.
DOI: 10.1016/j.energy.2005.11.002
Google Scholar
[2]
Rosen MA. Advances in hydrogen production by thermochemical water decomposition: a review. Energy 2010; 35: 1068-76.
Google Scholar
[3]
Wang ZL, Naterer GF, Gabriel KS, Gravelsins R, Daggupati VN: Comparison of sulfur-iodine and copper-chlorine thermochemical hydrogen production cycles. International Journal of Hydrogen Energy 2010; 35: 4820-30.
DOI: 10.1016/j.ijhydene.2009.09.006
Google Scholar
[4]
Naterer GF, Gabriel K, Wang ZL, Daggupati VN, Gravelsins R: Thermochemical hydrogen production with a copperchlorine cycle. I: oxygen release from copper oxychloride decomposition. International Journal of Hydrogen Energy 2008; 33: 5439-50.
DOI: 10.1016/j.ijhydene.2008.05.035
Google Scholar
[5]
Xu R, Wiesner T F: Dynamic model of a solar thermochemical water-splitting reactor with integrated energy collection and storage. International Journal of Hydrogen Energy 37 (2012) 2210-2223.
DOI: 10.1016/j.ijhydene.2011.10.053
Google Scholar
[6]
Zamfirescu C, Dincer I, Naterer GF. Thermophysical properties of copper compounds in copper-chlorine thermochemical water splitting cycles. International Journal of Hydrogen Energy 2010; 35: 4839-52.
DOI: 10.1016/j.ijhydene.2009.08.101
Google Scholar
[7]
Ghandehariun S, Naterer GF, Dincer I, Rosen MA. Solar thermochemical plant analysis for hydrogen production with the copper chlorine cycle. International Journal of Hydrogen Energy 2010; 35: 8511-20.
DOI: 10.1016/j.ijhydene.2010.05.028
Google Scholar
[8]
Lede J, Ferrer M. Solar thermochemical reactors. Journal de Physique IV 1999; 9: 253-8.
Google Scholar
[9]
Information on http: /www. MNRE. gov. in.
Google Scholar