p.909
p.913
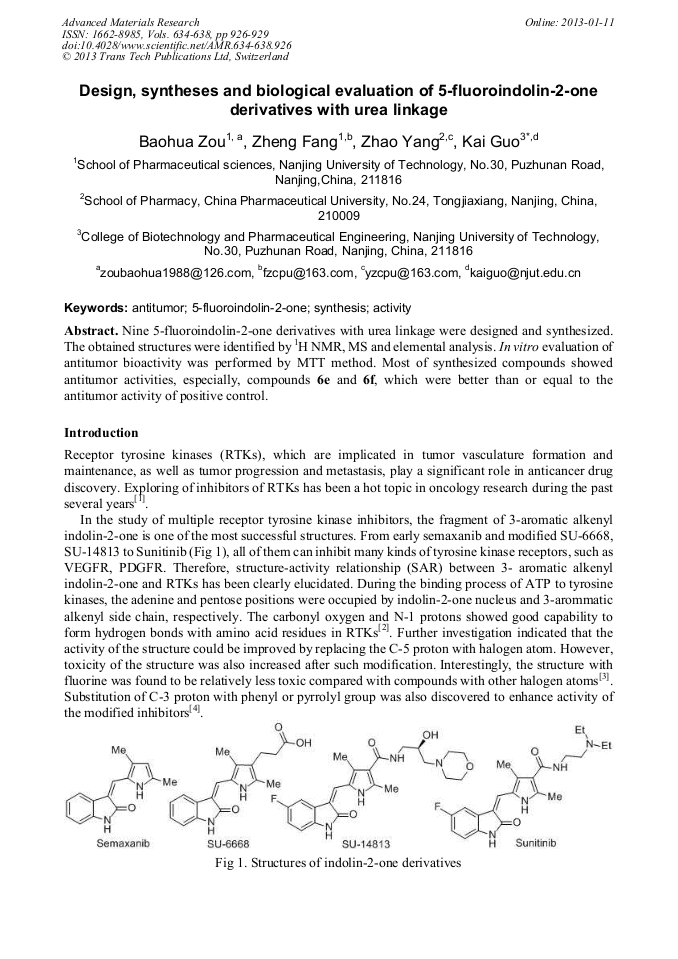
p.918
p.922
p.926
p.930
p.934
p.939
p.950
Design, Syntheses and Biological Evaluation of 5-Fluoroindolin-2-One Derivatives with Urea Linkage
Abstract:
Nine 5-fluoroindolin-2-one derivatives with urea linkage were designed and synthesized. The obtained structures were identified by 1H NMR, MS and elemental analysis. In vitro evaluation of antitumor bioactivity was performed by MTT method. Most of synthesized compounds showed antitumor activities, especially, compounds 6e and 6f, which were better than or equal to the antitumor activity of positive control.
Info:
Periodical:
Pages:
926-929
Citation:
Online since:
January 2013
Authors:
Keywords:
Price:
Сopyright:
© 2013 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: