p.1891
p.1896
p.1901
p.1907
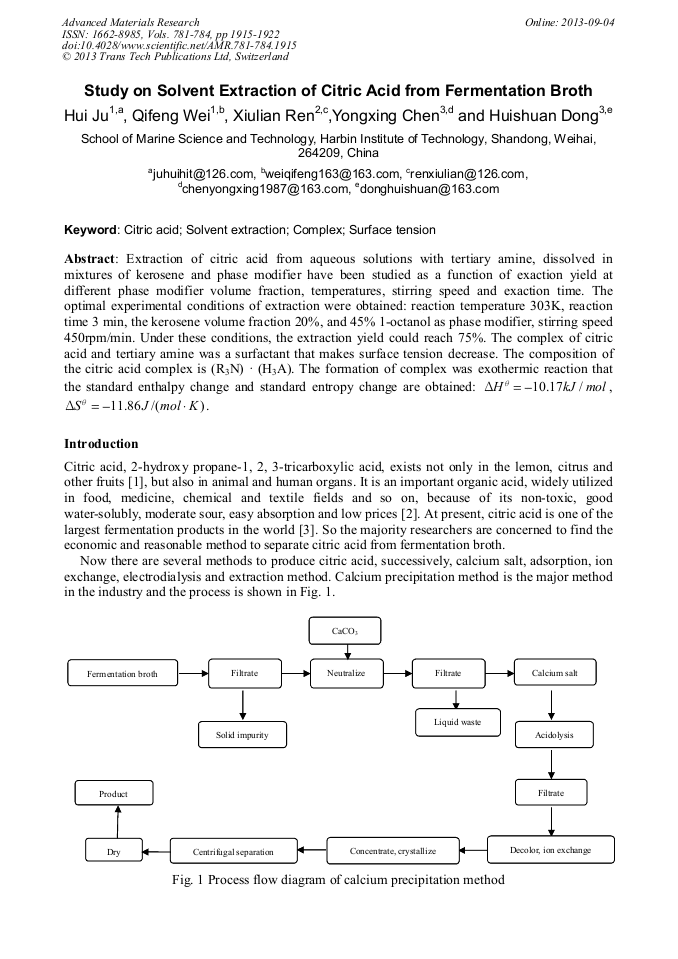
p.1915
p.1923
p.1931
p.1937
p.1941
Study on Solvent Extraction of Citric Acid from Fermentation Broth
Abstract:
Extraction of citric acid from aqueous solutions with tertiary amine, dissolved in mixtures of kerosene and phase modifier have been studied as a function of exaction yield at different phase modifier volume fraction, temperatures, stirring speed and exaction time. The optimal experimental conditions of extraction were obtained: reaction temperature 303K, reaction time 3 min, the kerosene volume fraction 20%, and 45% 1−octanol as phase modifier, stirring speed 450rpm/min. Under these conditions, the extraction yield could reach 75%. The complex of citric acid and tertiary amine was a surfactant that makes surface tension decrease. The composition of the citric acid complex is (R3N) · (H3A). The formation of complex was exothermic reaction that the standard enthalpy change and standard entropy change are obtained: ΔHθ=-10.17kJ/mol, ΔSθ=-11.86J/(mol·K).
Info:
Periodical:
Pages:
1915-1922
Citation:
Online since:
September 2013
Authors:
Keywords:
Price:
Сopyright:
© 2013 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: