p.811
p.815
p.819
p.823
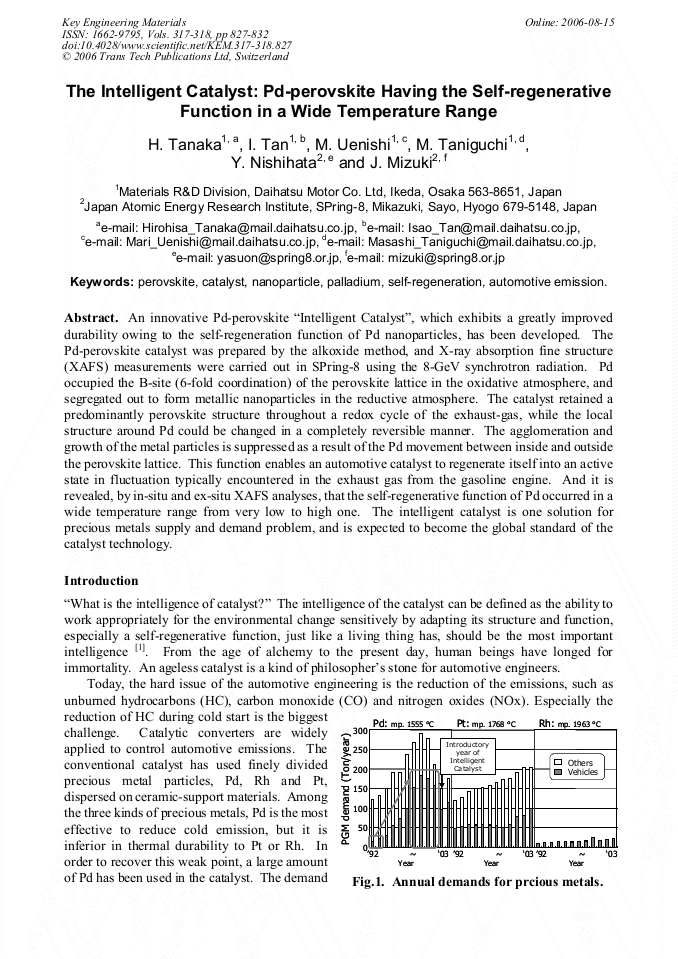
p.827
p.833
p.837
p.841
p.845
The Intelligent Catalyst: Pd-Perovskite Having the Self-Regenerative Function in a Wide Temperature Range
Abstract:
An innovative Pd-perovskite “Intelligent Catalyst”, which exhibits a greatly improved durability owing to the self-regeneration function of Pd nanoparticles, has been developed. The Pd-perovskite catalyst was prepared by the alkoxide method, and X-ray absorption fine structure (XAFS) measurements were carried out in SPring-8 using the 8-GeV synchrotron radiation. Pd occupied the B-site (6-fold coordination) of the perovskite lattice in the oxidative atmosphere, and segregated out to form metallic nanoparticles in the reductive atmosphere. The catalyst retained a predominantly perovskite structure throughout a redox cycle of the exhaust-gas, while the local structure around Pd could be changed in a completely reversible manner. The agglomeration and growth of the metal particles is suppressed as a result of the Pd movement between inside and outside the perovskite lattice. This function enables an automotive catalyst to regenerate itself into an active state in fluctuation typically encountered in the exhaust gas from the gasoline engine. And it is revealed, by in-situ and ex-situ XAFS analyses, that the self-regenerative function of Pd occurred in a wide temperature range from very low to high one. The intelligent catalyst is one solution for precious metals supply and demand problem, and is expected to become the global standard of the catalyst technology.
Info:
Periodical:
Pages:
827-832
Citation:
Online since:
August 2006
Keywords:
Price:
Сopyright:
© 2006 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: