p.227
p.231
p.235
p.239
p.243
p.247
p.251
p.255
p.259
Synthesis a New Function Materials and Photochromic Properties of Diarylethene Bearing a Naphthalene and Isoxazole Moiety
Abstract:
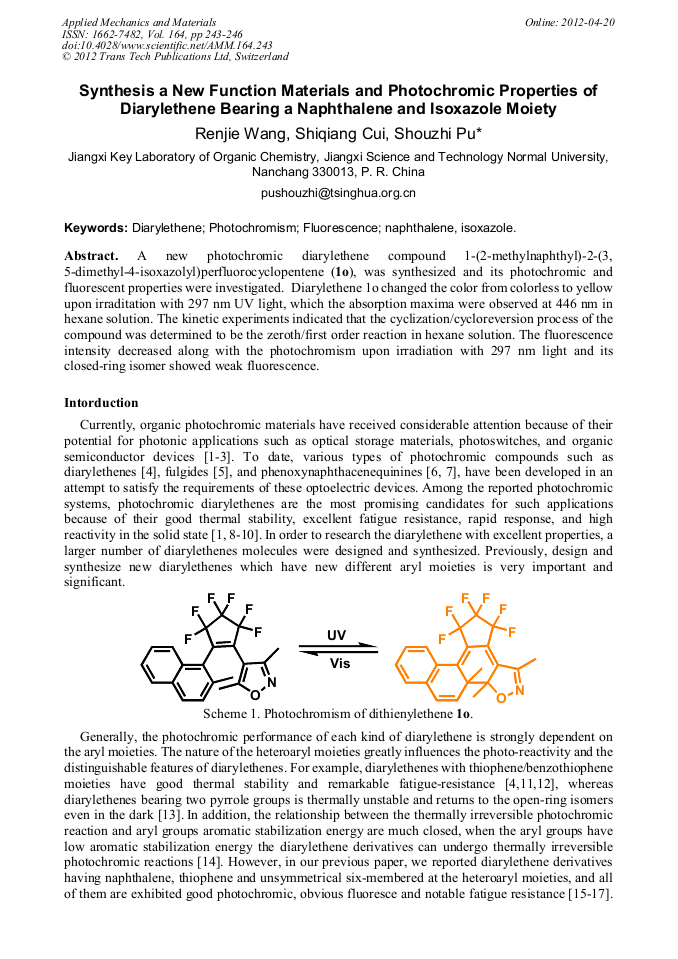
A new photochromic diarylethene compound 1-(2-methylnaphthyl)-2-(3, 5-dimethyl-4-isoxazolyl)perfluorocyclopentene (1o), was synthesized and its photochromic and fluorescent properties were investigated. Diarylethene 1o changed the color from colorless to yellow upon irraditation with 297 nm UV light, which the absorption maxima were observed at 446 nm in hexane solution. The kinetic experiments indicated that the cyclization/cycloreversion process of the compound was determined to be the zeroth/first order reaction in hexane solution. The fluorescence intensity decreased along with the photochromism upon irradiation with 297 nm light and its closed-ring isomer showed weak fluorescence
Info:
Periodical:
Pages:
243-246
DOI:
Citation:
Online since:
April 2012
Authors:
Keywords:
Price:
Сopyright:
© 2012 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: