p.231
p.235
p.239
p.243
p.247
p.251
p.255
p.259
p.263
Synthesis and Properties of a Novel Photochromic Diarylethene Material Bearing Two Five-Membered Aryl Unit
Abstract:
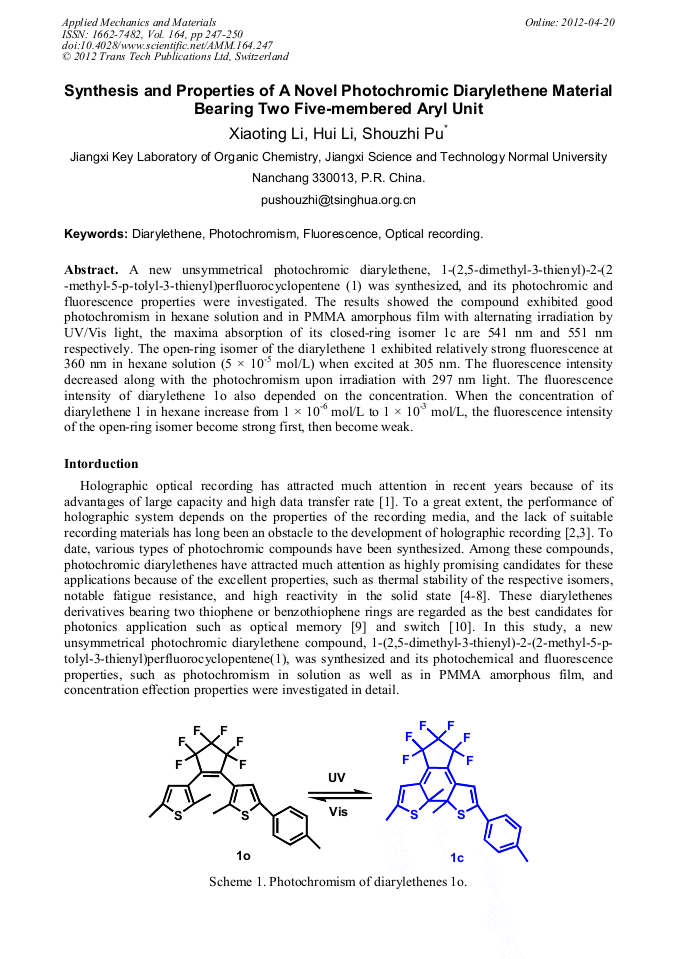
A new unsymmetrical photochromic diarylethene, 1-(2,5-dimethyl-3-thienyl)-2-(2 -methyl-5-p-tolyl-3-thienyl)perfluorocyclopentene (1) was synthesized, and its photochromic and fluorescence properties were investigated. The results showed the compound exhibited good photochromism in hexane solution and in PMMA amorphous film with alternating irradiation by UV/Vis light, the maxima absorption of its closed-ring isomer 1c are 541 nm and 551 nm respectively. The open-ring isomer of the diarylethene 1 exhibited relatively strong fluorescence at 360 nm in hexane solution (5 × 10-5 mol/L) when excited at 305 nm. The fluorescence intensity decreased along with the photochromism upon irradiation with 297 nm light. The fluorescence intensity of diarylethene 1o also depended on the concentration. When the concentration of diarylethene 1 in hexane increase from 1 × 10-6 mol/L to 1 × 10-3 mol/L, the fluorescence intensity of the open-ring isomer become strong first, then become weak
Info:
Periodical:
Pages:
247-250
DOI:
Citation:
Online since:
April 2012
Authors:
Keywords:
Price:
Сopyright:
© 2012 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: