p.451
p.455
p.459
p.462
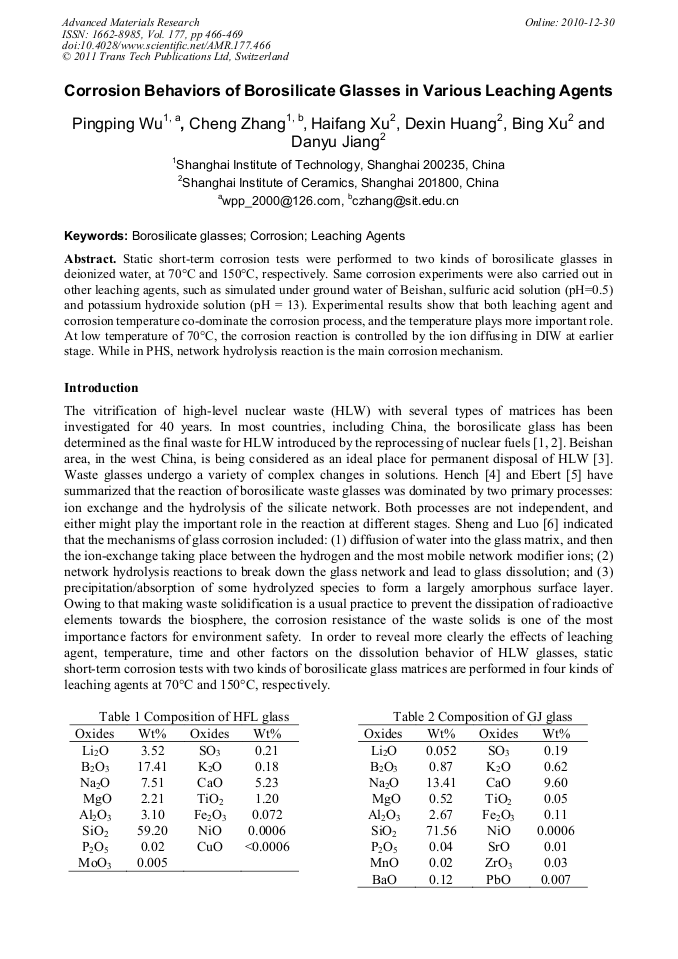
p.466
p.470
p.475
p.478
p.481
Corrosion Behaviors of Borosilicate Glasses in Various Leaching Agents
Abstract:
Static short-term corrosion tests were performed to two kinds of borosilicate glasses in deionized water, at 70°C and 150°C, respectively. Same corrosion experiments were also carried out in other leaching agents, such as simulated under ground water of Beishan, sulfuric acid solution (pH=0.5) and potassium hydroxide solution (pH = 13). Experimental results show that both leaching agent and corrosion temperature co-dominate the corrosion process, and the temperature plays more important role. At low temperature of 70°C, the corrosion reaction is controlled by the ion diffusing in DIW at earlier stage. While in PHS, network hydrolysis reaction is the main corrosion mechanism.
Info:
Periodical:
Pages:
466-469
DOI:
Citation:
Online since:
December 2010
Authors:
Keywords:
Price:
Сopyright:
© 2011 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: