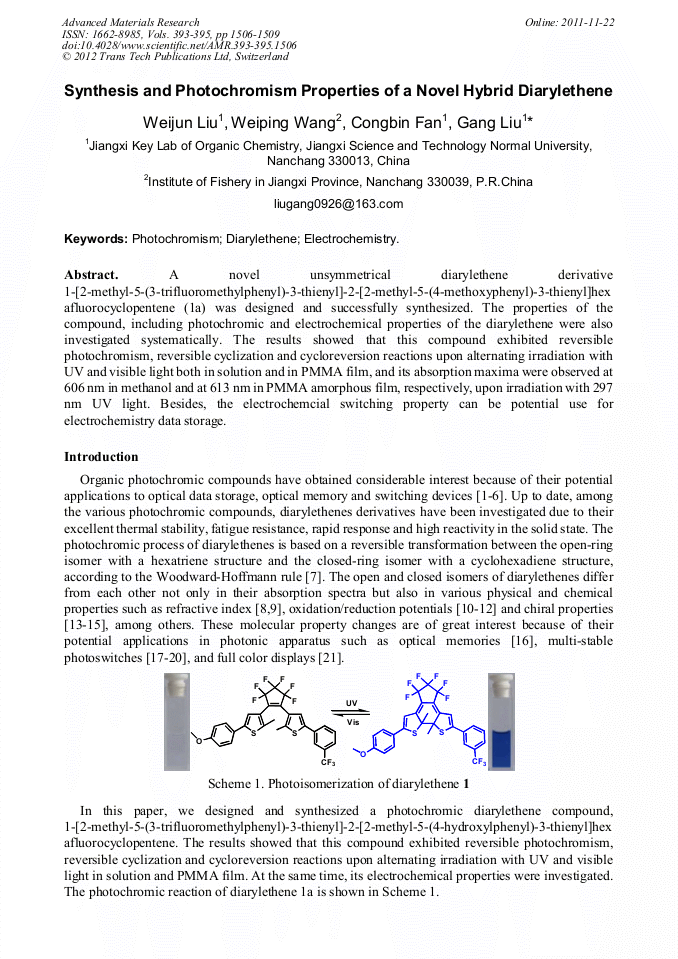
[1]
M. Irie: Photo-reactive Materials for Ultrahigh-density Optical Memory (Elsevier Science Ltd. Europe 1994).
Google Scholar
[2]
B.L. Feringa: Molecular Switches (Wiley-VCH: Weinheim, Germany 2001).
Google Scholar
[3]
M. Irie: Chem. Rev. vol. 100 (2000), p.1685.
Google Scholar
[4]
H. Tian and S.J. Yang: Chem. Soc. Rev. vol. 33 (2004), p.85.
Google Scholar
[5]
M. Irie, T. Fukaminato, T. Sasaki, N. Tamai and T. Kawai: Nature vol. 420 (2002), p.759.
DOI: 10.1038/420759a
Google Scholar
[6]
H. Durr and H. Bouus-Laurent: Photochromism: Molecules and Systems (Elsevier, Amsterdam The Netherlands, 1990).
Google Scholar
[7]
K. Matsuda and M. Irie: J. Photochem. Photobiol. C. vol. 5 (2004), p.169.
Google Scholar
[8]
C. Bertarelli, A. Bianco, F. D'Amore, M.C. Gallazzi and G. Zerbi: Adv. Funct. Mater. vol. 14 (2004), p.357.
Google Scholar
[9]
E. Kim, Y. -K. Choi and M. -H. Lee: Macromolecules, vol. 32 (1999), p.4855.
Google Scholar
[10]
G. Guirado, C. Coudret, M. Hliwa and J.P. Launay: J. Phys. Chem. B vol. 109 (2005), p.17445.
Google Scholar
[11]
W.R. Browne, J.J.D. de Jong, T. Kudernac, M. Walko, L.N. Lucas, K. Uchida, J.H. van Esch and B.L. Feringa: Chem. Eur. J. vol. 11 (2005), p.6414.
DOI: 10.1002/chem.200500162
Google Scholar
[12]
W.R. Browne, J.J.D. de Jong, T. Kudernac, M. Walko, L.N. Lucas, K. Uchida, J.H. van Esch and B.L. Feringa: Chem. Eur. J. vol. 11 (2005), 6430.
DOI: 10.1002/chem.200500163
Google Scholar
[13]
T. Okuyama, Y. Tani, K. Miyake and Y. Yokoyama: J. Org. Chem. vol. 72 (2007), p.1634.
Google Scholar
[14]
Y. Tani, T. Ubukata and Y. Yokoyama: J. Org. Chem. vol. 72 (2007), p.1639.
Google Scholar
[15]
K. Matsuda, S. Yamamoto and M. Irie: Tetrahedron Lett. Vol. 42 (2001), p.7291.
Google Scholar
[16]
C.C. Corredor, Z.L. Huang and K.D. Belfield: Adv. Mater. vol. 18 (2006), p.2910.
Google Scholar
[17]
H. Tian and Y.L. Feng: J. Mater. Chem. vol. 18 (2008), p.1617.
Google Scholar
[18]
J.J. Zhang, W.J. Tan, X.L. Meng and H. Tian: J. Mater. Chem. vol. 19 (2009), p.5726.
Google Scholar
[19]
H. Tian and S. Wang: Chem. Commun. (2007), p.781.
Google Scholar
[20]
Y.L. Feng, Y.L. Yan, S. Wang, W.H. Zhu, S.X. Qian and H. Tian: J. Mater. Chem. vol. 16 (2006), p.3685.
Google Scholar
[21]
K. Higashiguchi, K. Matsuda, N. Tanifuji and M. Irie: J. Am. Chem. Soc. vol. 127 (2005), p.8922.
Google Scholar
[22]
D. Bonifazi, M. Scholl, F. Song, L. Echegoyen, G. Accorsi, N. Armaroli and F. Diederich: Angew. Chem. Int. Ed. vol. 42 (2003), p.4966.
DOI: 10.1002/anie.200352265
Google Scholar
[23]
B. Gorodetsky, H. Samachetty, R.L. Donkers, M.S. Workentin and N.R. Branda: Angew. Chem. Int. Ed. vol. 43 (2004), p.2812.
DOI: 10.1002/anie.200353029
Google Scholar
[24]
X. W. Zhan, Y. Q. Liu, X. Wu, S. Wang and D. B. Zhu: Macromolecules, vol. 35 (2002), p.2529.
Google Scholar