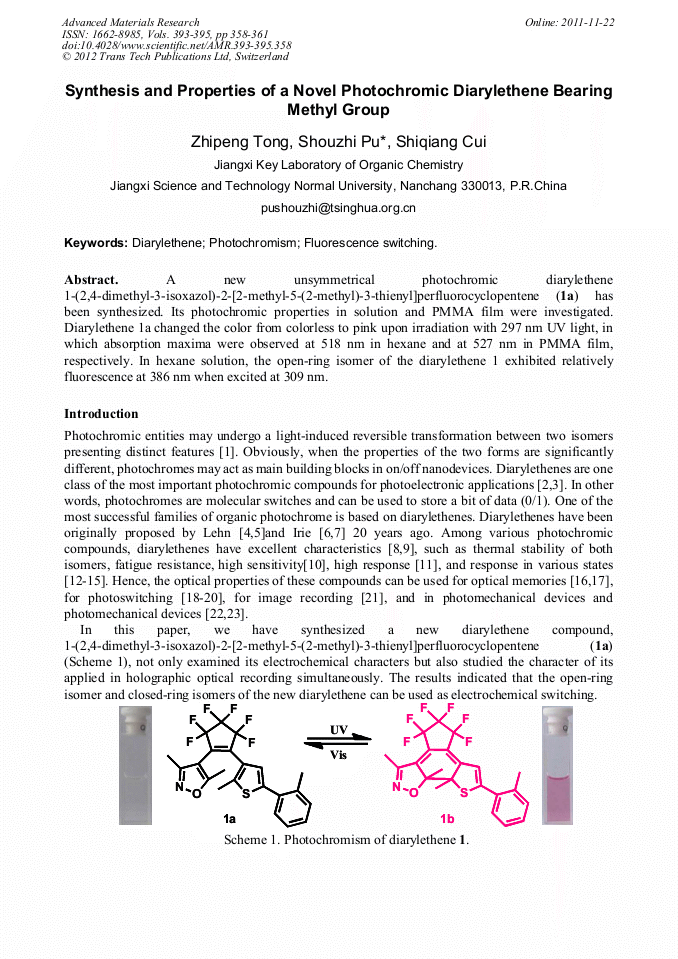
[1]
H. Durr, L. Bouas: H. Photochromism: Molecules and Systems ( Elsevier Publications, New York 2003).
Google Scholar
[2]
J.C. Crano, R.J. Guglielmetti, in: Organic Photochromic and Thermochromic Compounds, edited by Plenum Publications, New York (1999), in press.
Google Scholar
[3]
H. Durr, L. Bouas: H. Photochromism: Molecules and Systems ( Elsevier Publications, New York 1990).
Google Scholar
[4]
S.L. Gilat, S.H. Kawai and J.M. Lehn: J. Chem. Soc. Chem. Commun. (1993), p.1439.
Google Scholar
[5]
S.L. Gilat, S.H. Kawai and J.M. Lehn: Chem. —Eur. J. Forum Vol. 1(1995), p.275.
Google Scholar
[6]
M. Irie and M.J. Mohri: Org. Chem. Forum Vol. 53 (1988), p.803.
Google Scholar
[7]
M. Irie and K.J. Sayo: Phys. Chem. Forum Vol. 96 (1992), p.7671.
Google Scholar
[8]
M. Irie: Chem. ReV. Forum Vol. 100 (2000), p.1685.
Google Scholar
[9]
M. Irie and K. Bull. Uchida: Chem. Soc. Jpn. Forum Vol. 71 (1998), p.985.
Google Scholar
[10]
M. Irie, T. Lifka, S. Kobatake and N.J. Kato: Am. Chem. Soc. Forum Vol. 122 (2000), p.4871.
Google Scholar
[11]
H. Miyasaka, T. Nobuto, A. Itaya, N. Tamai and M. Irie: Chem. Phys. Lett. Forum Vol. 269 (1997), p.281.
Google Scholar
[12]
S. Kobatake and M. Irie: Bull. Chem. Soc. Jpn. Forum Vol. 77 (2004), p.195.
Google Scholar
[13]
T. Kawai, N. Fukuda, D. Gröschl, S. Kobatake and M. Irie: Jpn. J. Appl. Phys. Forum Vol. 38 (1999), p.1194.
Google Scholar
[14]
E. Kim, Y. Choi and M. Lee: Macromolecules. Forum Vol. 32 (1999), p.4855.
Google Scholar
[15]
H. Nakashima and M. Irie: Macromol. Chem. Phys. Forum Vol. 200 (1999), p.683.
Google Scholar
[16]
M. Irie: Jpn. J. Appl. Phys. Forum Vol. 28 (1989), p.215.
Google Scholar
[17]
T. Tsujioka, M. Kume and M. Irie: Jpn. J. Appl. Phys. Forum Vol. 34 (1995), p.6439.
Google Scholar
[18]
M. Takeshita, K. Uchida and M. Irie: Chem. Commun. (1996), p.1807.
Google Scholar
[19]
K. Matsuda and M. Irie: J. Am. Chem. Soc. Forum Vol. 122 (2000), p.7195.
Google Scholar
[20]
K. Matsuda, M. Matsuo and M. Irie: J. Org. Chem. Forum Vol. 66 (2001), p.8799.
Google Scholar
[21]
S. Kawata and Y. Kawata: Chem. ReV. Forum Vol. 100 (2000), p.1777.
Google Scholar
[22]
M. Irie, S. Kobatake and M. Horichi: Science. Forum Vol. 291 (2001), p.1769.
Google Scholar
[23]
S. Kobatake, S. Takami, H. Muto, T. Ishikawa and M. Irie: Nature. Forum Vol. 446 (2007), p.778.
Google Scholar
[24]
A. Spangenberg, R. Métivier, J. Gonzalez, K. Nakatani, P. Yu, M. Giraud, A. Léaustic, R. Guillot, T. Uwada and T. i Asahi: Adv. Mater. Vol. 21 (2009), p.309.
DOI: 10.1002/adma.200801578
Google Scholar
[25]
Z.X. Li, L.Y. Liao, S. Wei, C. Xu, Z. Chao, C. Fang and C. Yan: J. Phys. Chem. C. Vol. 112 (2008), p.5190.
Google Scholar
[26]
K. Kasatani, S. Kambe and M. Irie: J. Photoch. Photobiol. A. Vol. 122 (1999), p.11.
Google Scholar
[27]
B. Chen, M. Wang, Y. Wu and H. Tian: Chem. Commun. (2002), p.1060.
Google Scholar
[28]
H. Tian, B.Z. Chen, H.Y. Tu and K. Müllen: Adv. Mater. Vol. 14 (2002), p.918.
Google Scholar