p.1102
p.1107
p.1111
p.1115
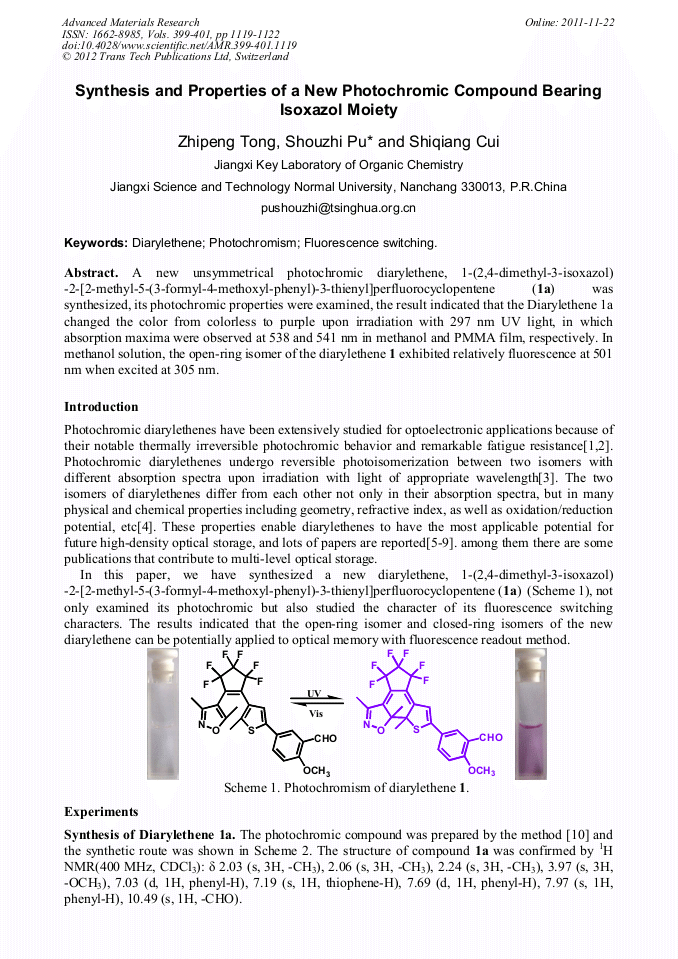
p.1119
p.1123
p.1131
p.1135
p.1139
Synthesis and Properties of a New Photochromic Compound Bearing Isoxazol Moiety
Abstract:
A new unsymmetrical photochromic diarylethene, 1-(2,4-dimethyl-3-isoxazol)-2-[2-methyl-5-(3- formyl-4- methoxyl)-3-thienyl]perfluorocyclopentene (1a) was synthesized, its photochromic properties were examined, the result indicated that the Diarylethene 1a changed the color from colorless to purple upon irradiation with 297 nm UV light, in which absorption maxima were observed at 538 and 541 nm in methanol and PMMA film, respectively. In methanol solution, the open-ring isomer of the diarylethene 1 exhibited relatively fluorescence at 501 nm when excited at 305 nm.
Info:
Periodical:
Pages:
1119-1122
Citation:
Online since:
November 2011
Authors:
Keywords:
Price:
Сopyright:
© 2012 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: