p.3110
p.3114
p.3118
p.3123
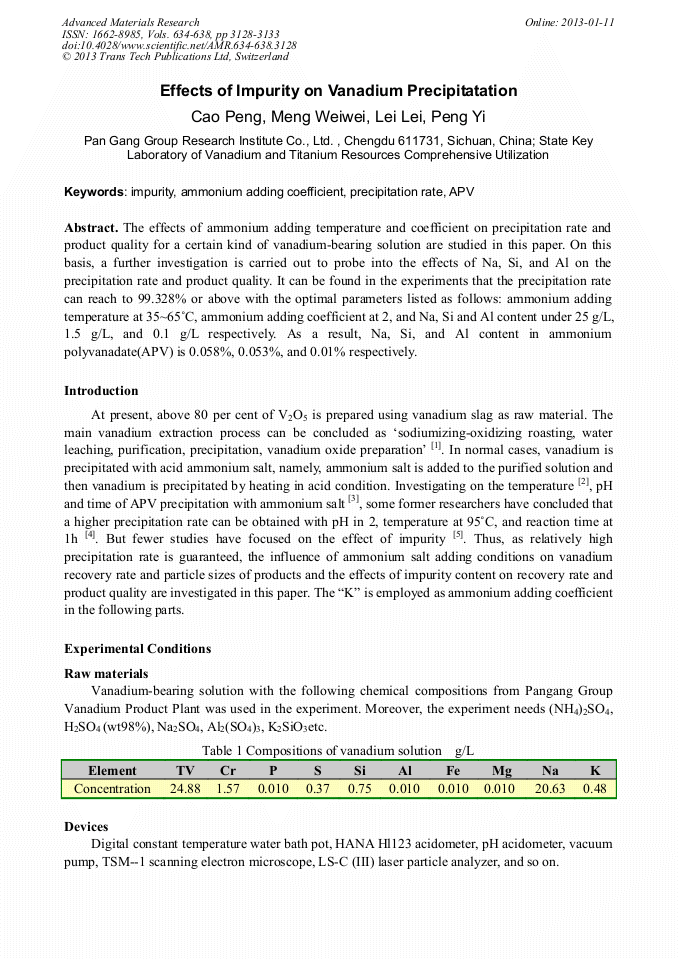
p.3128
p.3134
p.3138
p.3143
p.3149
Effects of Impurity on Vanadium Precipitatation
Abstract:
The effects of ammonium adding temperature and coefficient on precipitation rate and product quality for a certain kind of vanadium-bearing solution are studied in this paper. On this basis, a further investigation is carried out to probe into the effects of Na, Si, and Al on the precipitation rate and product quality. It can be found in the experiments that the precipitation rate can reach to 99.328% or above with the optimal parameters listed as follows: ammonium adding temperature at 35~65°C, ammonium adding coefficient at 2, and Na, Si and Al content under 25 g/L, 1.5 g/L, and 0.1 g/L respectively. As a result, Na, Si, and Al content in ammonium polyvanadate(APV) is 0.058%, 0.053%, and 0.01% respectively.
Info:
Periodical:
Pages:
3128-3133
Citation:
Online since:
January 2013
Authors:
Keywords:
Price:
Сopyright:
© 2013 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: