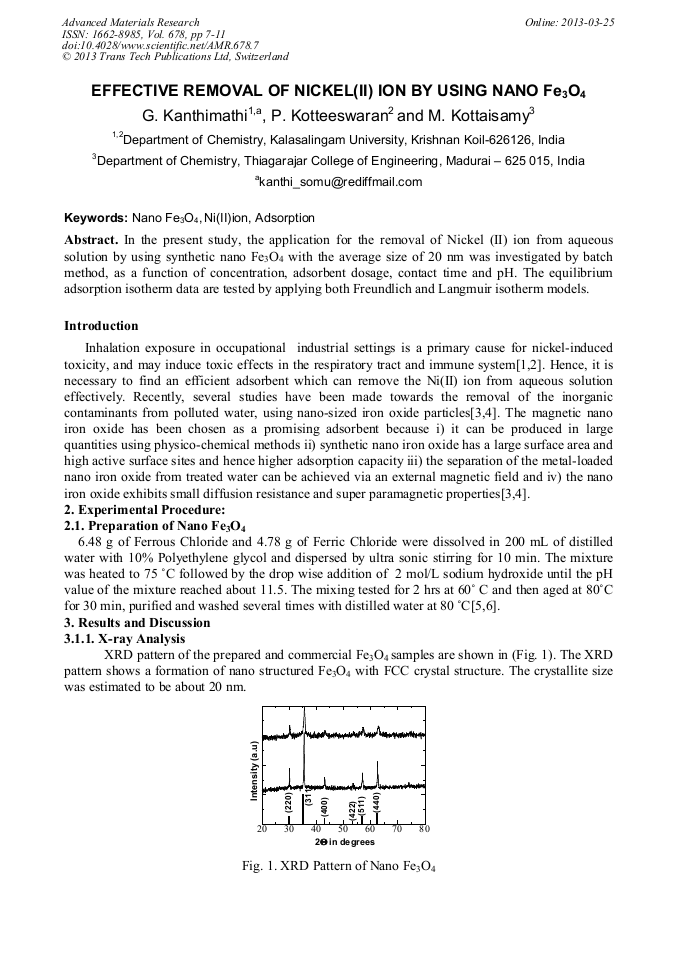
[1]
L. T. Haber, L. Erdreicht, G. L. Diamond, A. M. Maier, R. Ratney, Q. Zhao, M. L. Dourson, Hazard Identification and Dose Response of Inhaled Nickel-Soluble Salts, Ragul. Toxico.Pharmacol. 31 (2000) 210-230.
DOI: 10.1006/rtph.2000.1377
Google Scholar
[2]
P.B. Kelter, J. Grundman, D.S. Hage, J.D. Carr, C.M. Castro-Acuna, A discussion of water pollution in the united states and Mexico: with high school laboratory activities for the analysis of lead, atrzine, and nitrate, J. Chem. Educ. 74 (1997) 1413-1421.
DOI: 10.1021/ed074p1413
Google Scholar
[3]
Meng Xo, Yunsong Zhang, Zhiming Zhang, Yaou Shen, Maojun Zhao, Guangtang Pan, Study on the adsorption of Ca2+, Cd2+ and Pb2+ by magnetic Fe3O4 yeast treated with EDTA dianhydride, Chem. Eng. J. 168 (2011) 737-745.
DOI: 10.1016/j.cej.2011.01.069
Google Scholar
[4]
Z.L. Liu, H.B. Wang , H.Q. Lu, G.H. Du, L. Peng, Y.Q. Du, S.M. Zhang, K.L. Yao, Synthesis and characterization of Ultrafine well-dispersed magnetic nanoparticle, J.Magn.Mater. 283 (2004) 258-262.
DOI: 10.1016/j.jmmm.2004.05.031
Google Scholar
[5]
Yuanbi Zhao, Zuminqiu, Jiaying Hunag, Preparation and Analysis of Fe3O4 magnetic nanoparticles used as Targeted-drug carriers, Chin. J. Chem. Eng. 16 (2008) 451.
Google Scholar
[6]
Y.F. Shen, J. Tang, Z.H. Nie, Y.D. Wang, Y. Ren, L. Zuo, Preparation and application of magnetic Fe3O4 nanoparticles for waste water purification, Sep. Purif. Technol. 68 (2009) 312.
DOI: 10.1016/j.seppur.2009.05.020
Google Scholar
[7]
L. Panda, B. Das, D.S. Rao, B.K. Mishra, Application of dolochar in the removal of cadmium and hexavalent chromium ions from aqueous solutions, J. Hazard. Mater. 192 (2011) 822-831.
DOI: 10.1016/j.jhazmat.2011.05.098
Google Scholar
[8]
Y.F. Shen, J. Tang, Z.H. Nie, Y.D. Wang, Y. Ren, L. Zuo, Preparation and application of magnetic Fe3O4 nanoparticles for waste water purification, Sep. Purif. Technol. 68 (2009) 312-319.
DOI: 10.1016/j.seppur.2009.05.020
Google Scholar
[9]
Yang-Chuang Chang, Dong-Hwang Chen, Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu(II) ions, J.Colloid and Interface Sci. 283 (2005) 446-451.
DOI: 10.1016/j.jcis.2004.09.010
Google Scholar
[10]
S.C. Malhotra and P.C. Kanna, Ind. Env. Health. 19 (1997) 224.
Google Scholar