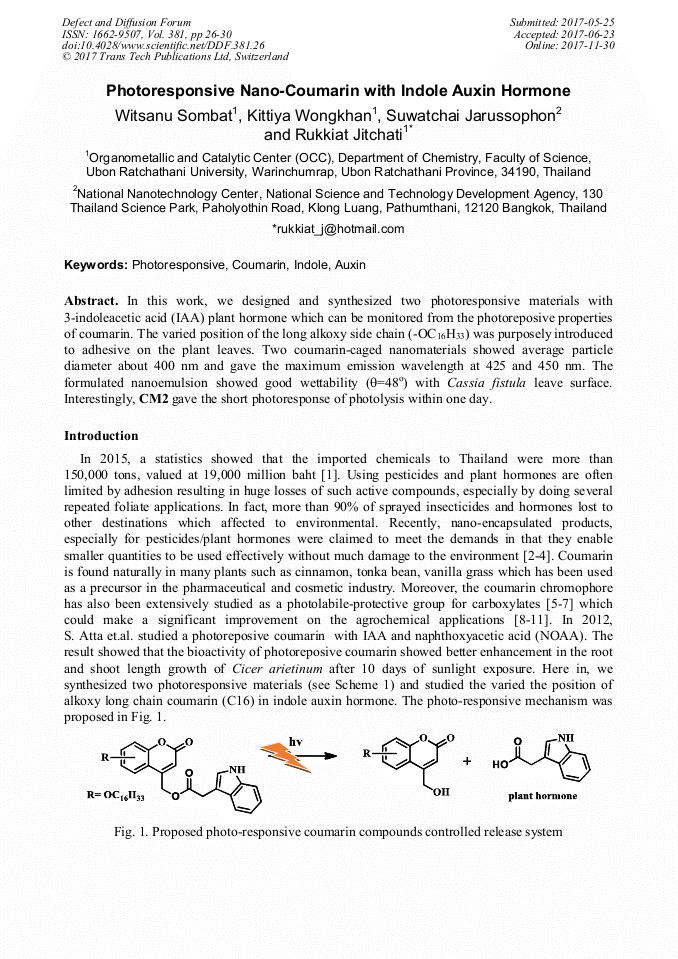
[1]
Information on http: /www. doa. go. th/ard.
Google Scholar
[2]
A. Jana, B. Saha, D.R. Banerjee, S.K. Ghosh, K.T. Nguyen, X. Ma, Q. Qu, Y. Zhao, N.P. Singh, Photocontrolled Nuclear-Targeted Drug Delivery by Single Component Photoresponsive Fluorescent Organic Nanoparticles of Acridin-9-Methanol, Bioconjugate Chem. 24 (2013).
DOI: 10.1021/bc400170r
Google Scholar
[3]
A. Jana, K.S.P. Devi, T.K. Maiti, N.P. Singh, Perylene-3-ylmethanol: Fluorescent Organic Nanoparticles as a Single-Component Photoresponsive Nanocarrier with Real Time Monitoring of Anticancer Drug Release, J. Am. Chem. Soc. 134 (2012) 7656-7659.
DOI: 10.1021/ja302482k
Google Scholar
[4]
S. Karthik, N. Puvvada, B.P. Kumar, S. Rajput, A. Pathak, M. Mandal, N.P. Singh, Photoresponsive Coumarin-Tethered Multifunctional Magnetic Nanoparticles for Release of Anticancer Drug, ACS Appl. Mater. Interfaces. 5 (2013) 5232-5238.
DOI: 10.1021/am401059k
Google Scholar
[5]
R. Subramaniam, Y. Xiao, Y. Li, S.Y. Qian, W. Sun, S. Mallik, Light-mediated and H-bond facilitated liposomal release: the role of lipid head groups in release efficiency, Tetrahedron Lett. 51 (2010) 529–532.
DOI: 10.1016/j.tetlet.2009.11.084
Google Scholar
[6]
S. Atta, M. Ikbal, A. Kumar, N.P. Singh, Application of photoremovable protecting group for controlled release of plant growth regulators by sunlight, J. Photochem. Photobiol. B, Biol. 111 (2012) 39-49.
DOI: 10.1016/j.jphotobiol.2012.03.008
Google Scholar
[7]
P. Bourbon, Q. Peng, G. Ferraudi, C. Stauffacher, O. Wiest, P. Helquist, Synthesis and photochemical behavior of coumarin-caged Cholesterol, Bioorg. Med. Chem. Lett. 23 (2013) 2162-2165.
DOI: 10.1016/j.bmcl.2013.01.095
Google Scholar
[8]
M. Kah, S. Beulke, K. Tiede, T. Hofmann, Nanopesticides: State of Knowledge, Environmental Fate, and Exposure Modeling, Crit Rev Environ Sci Technol. 43 (2013) 1823-1867.
DOI: 10.1080/10643389.2012.671750
Google Scholar
[9]
Q. Lin, C. Bao, G. Fan, S. Cheng, H. Liu, Z. Liu, L. Zhu, 7-Amino coumarin based fluorescent phototriggers coupled with nano/bio-conjugated bonds: Synthesis, labeling and photorelease, J. Mater. Chem. 22 (2012) 6680-6688.
DOI: 10.1039/c2jm30357d
Google Scholar
[10]
S. Atta, A. Jana, R. Ananthakirshnan, and P. S. N. Dhuleep, Fluorescent Caged Compounds of 2, 4-Dichlorophenoxyacetic Acid (2, 4-D): Photorelease Technology for Controlled Release of 2, 4-D, J. Agric. Food. Chem. 58 (2010) 11844–11851.
DOI: 10.1021/jf1027763
Google Scholar
[11]
S. Atta, A. Paul, R. Banerjee, M. Bera, M. Ikbal, D. Dhara, N.P. Singh, Photoresponsive polymers based on a coumarin moiety for the controlled release of pesticide 2, 4-D, RSC Advances. 5 (2015) 99968-99975.
DOI: 10.1039/c5ra18944f
Google Scholar