p.37
p.41
p.45
p.49
p.53
p.57
p.61
p.65
p.69
Synthesis and Lithium Ion Conductivity of MP2O7-Based Solid Electrolytes
Abstract:
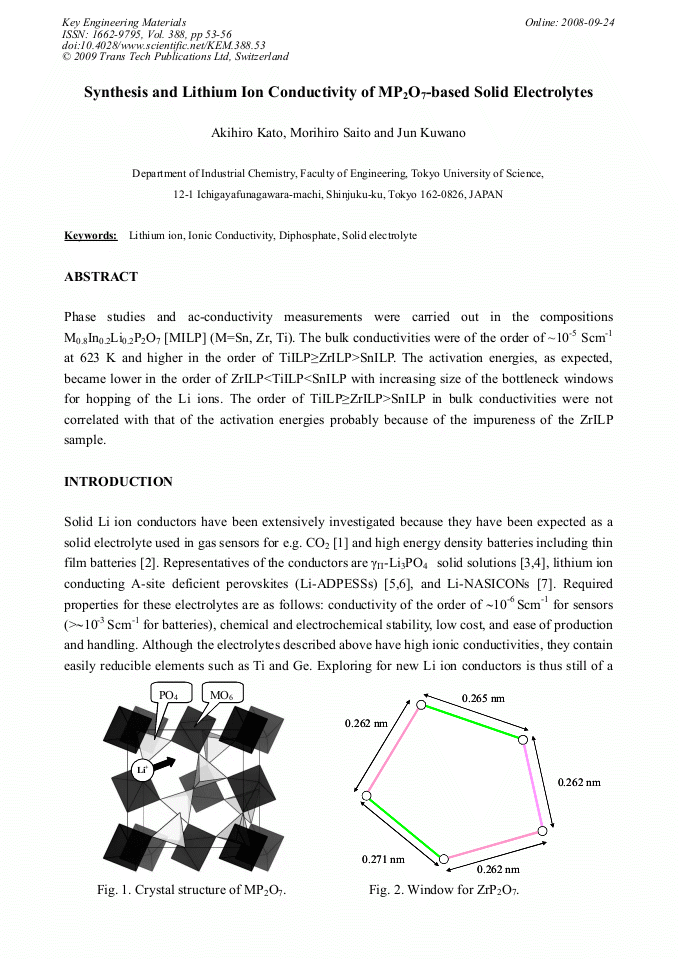
Phase studies and ac-conductivity measurements were carried out in the compositions
M0.8In0.2Li0.2P2O7 [MILP] (M=Sn, Zr, Ti). The bulk conductivities were of the order of ~10-5 Scm-1
at 623 K and higher in the order of TiILP≥ZrILP>SnILP. The activation energies, as expected,
became lower in the order of ZrILP
Info:
Periodical:
Pages:
53-56
DOI:
Citation:
Online since:
September 2008
Authors:
Keywords:
Price:
Сopyright:
© 2009 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: