p.368
p.372
p.377
p.381
p.386
p.390
p.395
p.399
p.404
Influence of Temperature on the Order-Disorder Transition in Gd2Zr2O7
Abstract:
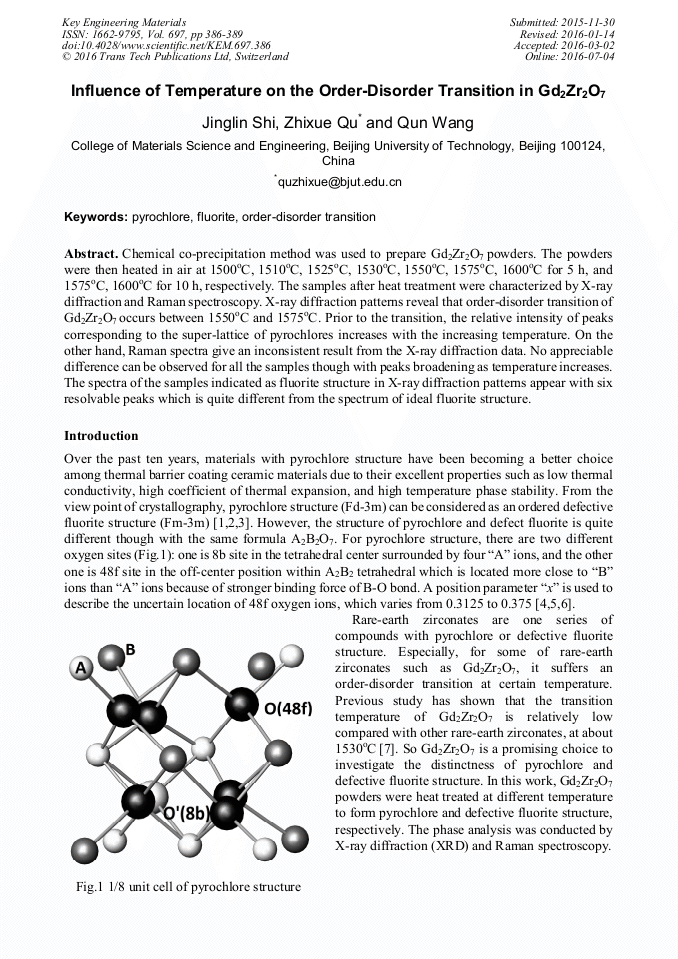
Chemical co-precipitation method was used to prepare Gd2Zr2O7 powders. The powders were then heated in air at 1500°C, 1510°C, 1525°C, 1530°C, 1550°C, 1575°C, 1600°C for 5 h, and 1575°C, 1600°C for 10 h, respectively. The samples after heat treatment were characterized by X-ray diffraction and Raman spectroscopy. X-ray diffraction patterns reveal that order-disorder transition of Gd2Zr2O7 occurs between 1550°C and 1575°C. Prior to the transition, the relative intensity of peaks corresponding to the super-lattice of pyrochlores increases with the increasing temperature. On the other hand, Raman spectra give an inconsistent result from the X-ray diffraction data. No appreciable difference can be observed for all the samples though with peaks broadening as temperature increases. The spectra of the samples indicated as fluorite structure in X-ray diffraction patterns appear with six resolvable peaks which is quite different from the spectrum of ideal fluorite structure.
Info:
Periodical:
Pages:
386-389
DOI:
Citation:
Online since:
July 2016
Authors:
Keywords:
Price:
Сopyright:
© 2016 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: