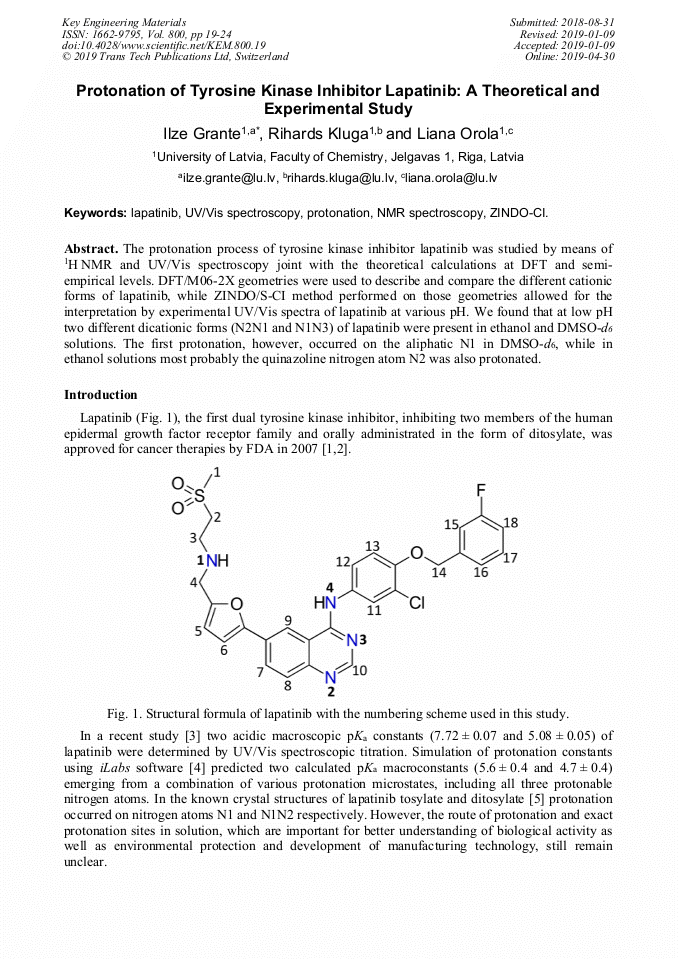
[1]
P.J. Medina, S. Goodin, Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases, Clin. Ther. 30 (2008) 1426-1447.
DOI: 10.1016/j.clinthera.2008.08.008
Google Scholar
[2]
M.H. Nelson, C.R. Dolder, Lapatinib: A novel dual tyrosine kinase inhibitor with activity in solid tumors, Ann. Pharmacother. 40 (2006) 261-269.
DOI: 10.1345/aph.1g387
Google Scholar
[3]
G. Tóth et al., Physicochemical characterization and cyclodextrin complexation of the anticancer drug lapatinib, J. Chem. 2017 (2017) 1-9.
Google Scholar
[4]
Information from http://www.acdlabs.com/ilab/. ACD/Labs, Advanced Chemistry Development Inc., 90 Adelaide Street, West Toronto, Ontario, M5H3V9, Canada.
DOI: 10.1021/ja040946z
Google Scholar
[5]
K. Ravikumar, B. Sridhar, N.J. Babu, A.K.S. Bhujanga Rao, R. Jyothiprasad, Tosylate salts of the anticancer drug lapatinib, Acta Crystallogr. Sect. C Cryst. Struct. Commun. 69 (2013) 1516-1523.
DOI: 10.1107/s0108270113028965
Google Scholar
[6]
I. Grante, A. Actins, L. Orola, Protonation effects on the UV/Vis absorption spectra of imatinib: A theoretical and experimental study, Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 129 (2014) 326-332.
DOI: 10.1016/j.saa.2014.03.059
Google Scholar
[7]
H. Kim, C.R. Babu, D.J. Burgess, Quantification of protonation in organic solvents using solution NMR spectroscopy: Implication in salt formation, Int. J. Pharm. 448 (2013) 123-131.
DOI: 10.1016/j.ijpharm.2013.03.040
Google Scholar
[8]
Gaussian 09 Revision-D.02. Gaussian, Inc., Wallingford CT, (2009).
Google Scholar
[9]
Y. Zhao, D.G. Truhlar, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function, Theor Chem Acc. 120 (2008) 215-241.
DOI: 10.1007/s00214-007-0310-x
Google Scholar
[10]
HyperChemProfessional, 7.51; Hypercube, Inc.: Gainesville, FL, (2007).
Google Scholar
[11]
G. Tóth et al., Physicochemical characterization and cyclodextrin complexation of erlotinib, Supramol. Chem. 28 (2015) 656-664.
Google Scholar
[12]
A.H. Fawcett, S. Fee, M. Stuckey, P. Walkden, The vibrational spectra and rotational isomerism of methyl ethyl sulphone-d0, -d3 and –d5, Spectrochim. Acta 43A, (1987) 797-804.
DOI: 10.1016/0584-8539(87)80222-2
Google Scholar
[13]
J. Scheigetz, C. Berthelette, C. Li, R.J. Zamboni, Base-catalyzed deuterium and tritium labeling of aryl methyl sulfones, J. Label. Compd. Radiopharm. 47 (2004) 881-880.
DOI: 10.1002/jlcr.880
Google Scholar
[14]
F.G. Bordwell, Equilibrium acidities in dimethyl sulfoxide solution, Acc. Chem. Res. 21 (1988) 456-463.
DOI: 10.1021/ar00156a004
Google Scholar
[15]
F.G. Bordwell, J.C. Branca, D.L. Hughes, W.N. Olmstead, Equilibria involving organic anions in dimethyl sulfoxide and N-mathylpyrrolidin-2-one: acidities, ion pairing, and hydrogen bonding, J. Org. Chem. 45 (1980) 3305-3313.
DOI: 10.1021/jo01304a034
Google Scholar