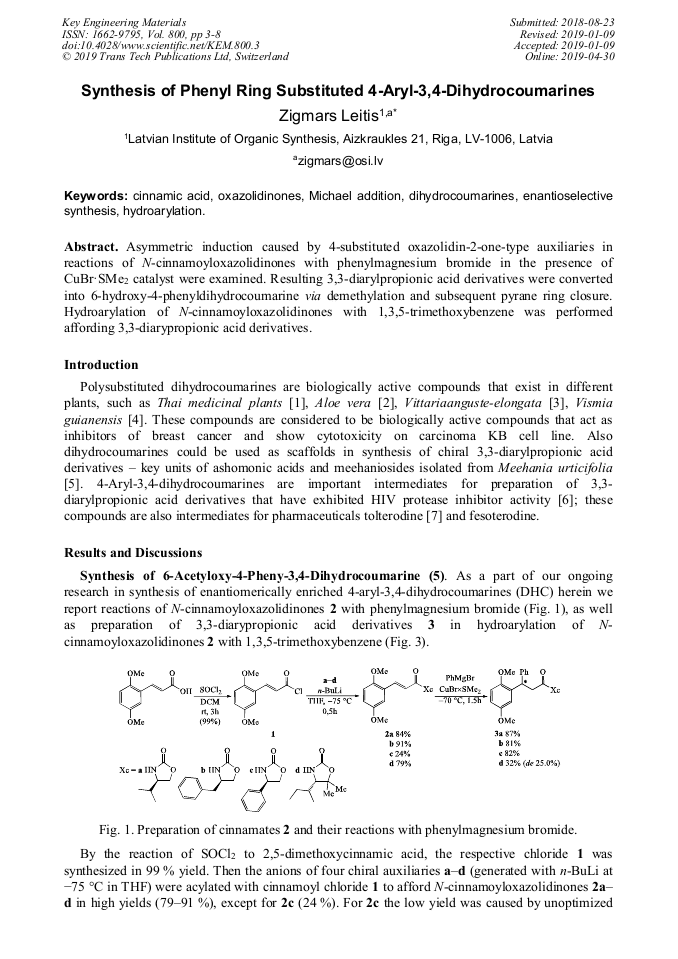
[1]
B. Wungsintaweekul, K. Umehara, T. Miyase, H. Noguchi, Estrogenic and anti-estrogenic compounds from the Thai medicinal plant, Smilax corbularia (Smilacaceae), Phytochemistry. 72 (2011) 495-502.
DOI: 10.1016/j.phytochem.2010.12.018
Google Scholar
[2]
X.F. Zhang, H.M. Wang, Y.L. Song, L.H. Nie, L.F. Wang, B. Lui, P.P. Shen, Y. Lui, Isolation, structure elucidation, antioxidative and immunomodulatory properties of two novel dihydrocoumarins from Aloe vera, Bioorg. Med. Chem. Lett. 16 (2006) 949-953.
DOI: 10.1016/j.bmcl.2005.10.096
Google Scholar
[3]
P.L. Wu, Y.L. Hsu, C.W. Zao, A.G. Damu, T.S. Wu, Constituents of vittaria anguste-elongata and their biological activities, J. Nat. Prod. 68 (2005) 1180-1184.
DOI: 10.1021/np050060o
Google Scholar
[4]
E.K. Seo, M.C. Wani, M.E. Wall, H. Navarro, R. Mukherjee, N.R. Farnsworth, A.D. Kinghorn, New bioactive aromatic compounds from Vismia guianensis, Phytochemistry. 55 (2000) 35-42.
DOI: 10.1016/s0031-9422(00)00208-9
Google Scholar
[5]
T. Murata, T. Miyase, F. Yoshizaki, Hyaluronidase inhibitory rosmarinic acid derivatives from meehania urticifolia, Chem. Pharm. Bull. 59 (2011) 88-95.
DOI: 10.1248/cpb.59.88
Google Scholar
[6]
P.D. Williams, J.A. McCauley, D.J. Bennett, C.J. Bungard, L. Chang, X.J. Chu, M.P. Dwyer, M.K. Holloway, K.M. Keertikar, H.M. Loughran, J.J. Manikowski, G.J. Morriello, D.M. Shen, E.C. Sherer, J. Schulz, S.T. Waddell, C.M. Wiscount, N. Zorn, S. Tummanapalli, V. Sivalenka, B. Hu, T. Ji, B. Zhong, WO Patent 2015013835. (2015).
DOI: 10.1021/acsmedchemlett.7b00386
Google Scholar
[7]
P.G. Gillberg, S. Sundquist, L. Nilvebrant, Comparison of the in vitro and in vivo profiles of tolterodine with those of subtype-selective muscarinic receptor antagonists, Eur. J. Pharmacol. 349 (1998) 285-292.
DOI: 10.1016/s0014-2999(98)00214-3
Google Scholar
[8]
Z. Leitis, V. Lusis, Conjugate addition of aryl nucleophiles to a,b-unsaturated cinnamic acid derivatives containing Evans type chiral auxiliaries, Tetrahedron: Asymmetry. 27 (2016) 843-851.
DOI: 10.1016/j.tetasy.2016.07.003
Google Scholar