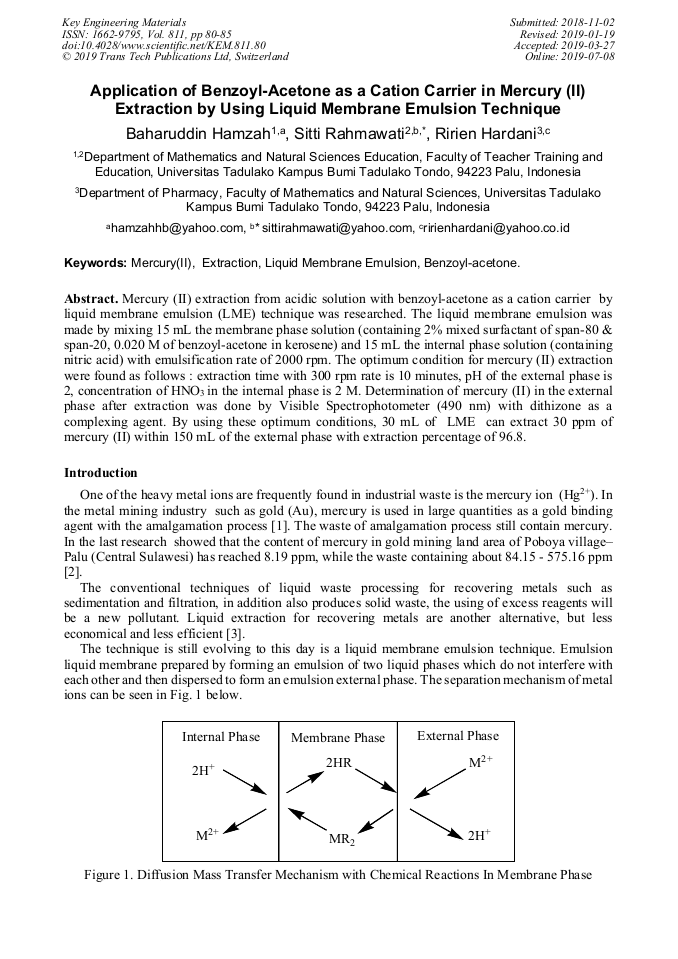
[1]
Jensen, M.,I., Bateman, A.M., 1981, Economic Mineral Deposits, 3rd Edition, John Wiley & Sons, New York, 593.
Google Scholar
[2]
Mirdat, S., Pata'dungan, Y.S. and Isrun, B., 2013. Status logam berat merkuri (Hg) dalam tanah pada kawasan pengolahan tambang emas di kelurahan Poboya, Kota Palu. AGROTEKBIS, 1(2), pp.127-134.
DOI: 10.22487/2540766x.2013.v10.i1.7456
Google Scholar
[3]
Hamzah, B., Jalaluddin, N., Wahab, A.W. and Upe, A., 2011. Pengaruh ion kadmium (II) dan nikel (II) pada ekstraksi ion tembaga (II) dengan ekstraktan 4-benzoil-1-fenil-3-metil-2-pirazolin-5-on menggunakan emulsi membran cair. Jurnal Natur Indonesia, 13(03), pp.269-275.
DOI: 10.31258/jnat.13.3.269-275
Google Scholar
[4]
Park, S.W., Kim, G.W., Kim, S.S. and Sohn, I.J., 2001. Facilitated transport of Cr (VI) through a supported liquid membrane with trioctylmethylammonium chloride as a carrier. Separation Science and Technology, 36(10), pp.2309-2326.
DOI: 10.1081/ss-100105920
Google Scholar
[5]
Kozlowski, C., Apostoluk, W., Walkowiak, W. and Kita, A., 2002. Removal of Cr (VI), Zn (II) and Cd (II) ions by transport across polymer inclusion membranes with basic ion carriers. Fizykochemiczne Problemy Mineralurgii, 36, pp.115-122.
DOI: 10.1081/ss-200038322
Google Scholar
[6]
Bourenane, S., Samar, M.E.H. and Abbaci, A., 2003. Extraction of cobalt and lead from waste water using a liquid surfactant membrane emulsion. Acta Chimica Slovenica, 50(4), pp.663-676.
Google Scholar
[7]
Basualto, C., Poblete, M., Marchese, J., Ochoa, A., Acosta, A., Sapag, J. and Valenzuela, F., 2006. Extraction of cadmium from aqueous solutions by emulsion liquid membranes using a stirred transfer cell contactor. Journal of the Brazilian Chemical Society, 17(7), pp.1347-1354.
DOI: 10.1590/s0103-50532006000700023
Google Scholar
[8]
Sabry, R., Hafez, A., Khedr, M. and El-Hassanin, A., 2007. Removal of lead by an emulsion liquid membrane: Part I. Desalination, 212(1-3), pp.165-175.
DOI: 10.1016/j.desal.2006.11.006
Google Scholar
[9]
Park, Y., Skelland, A.H.P., Forney, L.J. and Kim, J.H., 2006. Removal of phenol and substituted phenols by newly developed emulsion liquid membrane process. Water research, 40(9), pp.1763-1772.
DOI: 10.1016/j.watres.2006.03.005
Google Scholar
[10]
Luan, J. and Plaisier, A., 2004. Study on treatment of wastewater containing nitrophenol compounds by liquid membrane process. Journal of Membrane Science, 229(1-2), pp.235-239.
DOI: 10.1016/j.memsci.2003.08.019
Google Scholar
[11]
Chakravarti, A.K., Chowdhury, S.B. and Mukherjee, D.C., 2000. Liquid membrane multiple emulsion process of separation of copper (II) from waste waters. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 166(1-3), pp.7-25.
DOI: 10.1016/s0927-7757(99)00452-5
Google Scholar
[12]
Mohamed, Y.T. and Ibrahim, A.M., 2012. Extraction of copper from waste solution using liquid emulsion membrane. Journal of Environmental Protection, 3(01), pp.129-134.
DOI: 10.4236/jep.2012.31016
Google Scholar
[13]
Hamzah, B., Tuljannah, N. and Diharnaini, D., 2013, Ekstraksi Ion Tembaga (ii) Dengan Emulsi Membran Cair Menggunakan Ditizon Sebagai Pembawa Kation. Jurnal Akademika Kimia, 2(2), pp.76-81.
DOI: 10.30872/jkm.v15i1.493
Google Scholar
[14]
Manea, F., Dalea, V., Masu, S. and Negrea, P. 2003. Separation of Co (II) and Ni (II) ions from wastewater by emulsion liquid membrane technique. Sixth International Symposium and Exhibition on Environmental Contamination in Central and Eastern Europe, Prague, p.388.
Google Scholar
[15]
Hamzah, B., 2010. Aplikasi 1-fenil-3-metil-4-benzoil-5-pirazolon sebagai pembawa kation pada ekstraksi ion tembaga (II) menggunakan teknik emulsi membran cair. Disertasi Doktor Kimia pada Universitas Hasanuddin Makassar, Tidak Diterbitkan.
DOI: 10.31258/jnat.13.3.269-275
Google Scholar
[16]
Wang, S., He, P., Hao, D. and Zhu, Y., 2002. Treatment of Zn-containing acidic waste water by emulsion liquid membrane process. Tsinghua Science and Technology, 7(1), pp.91-94.
Google Scholar
[17]
Saravanan, S., Begum, K.M.S. and Anantharaman, N., 2006. Removal of hexavalent chromium by emulsion liquid membrane technique. Journal of the University of Chemical Technology and Metallurgy, 41(3), pp.333-342.
Google Scholar
[18]
Alam, S., Hamzah, B., Nuryanti, S. and Nurbaya, S., 2014. Penentuan kondisi optimum ekstraksi ion timbal (II) menggunakan teknik emulsi membran cair. Jurnal Akademika Kimia, 3(2), pp.104-110.
DOI: 10.22487/j24775185.2017.v6.i3.9443
Google Scholar
[19]
Djasman, S.M., Hamzah, B. and Husnia, H., 2014, Variasi Konsentrasi Span-80 Dan pH Fasa Eksternal pada Ekstraksi Ion Timbal (II) Dengan Metode Emulsi Membran Cair. Jurnal Akademika Kimia, 3(4), pp.192-197.
DOI: 10.22487/j24775185.2017.v6.i4.9454
Google Scholar
[20]
Hamzah, B., Astuti, W., Suherman, S. and Laonu, S.R., 2015, Variasi Perbandingan Volume Fasa Membran Dan Fasa Internal Serta Konsentrasi HNO3 Dalam Fasa Internal Terhadap Ekstraksi Ion Timbal (II) Menggunakan Teknik Emulsi Membran Cair. Jurnal Akademika Kimia, 4(2), pp.104-109.
DOI: 10.22487/j24775185.2017.v6.i3.9439
Google Scholar
[21]
Irawati, D.A., Hamzah, B. and Rahman, N., 2015, Pengaruh Ion Logam Cu (II) Terhadap Persen Ekstraksi Ion Pb (II) Menggunakan Teknik Emulsi Membran Cair. Jurnal Akademika Kimia, 5(4), pp.172-177.
DOI: 10.22487/j24775185.2016.v5.i4.8066
Google Scholar
[22]
Hamzah, B., Mucthar, H. and Rahmawati, S., 2017. Pengaruh Tembaga (II) dan Kadmium (II) Terhadap Persen Ekstraksi Merkuri (II) Menggunakan Emulsi Membran Cair Tipe W/O Bersurfaktan Ganda dengan Benzoil Aseton Sebagai Pembawa Kation. Jurnal Kimia Mulawarman, 15(1), pp.19-23.
DOI: 10.30872/jkm.v15i1.493
Google Scholar
[23]
Djunaidi, M.C., Lusiana, R.A., Wibawa, P.J., Siswanta, D. and Jumina, J., 2010. Sintesis Turunan Polieugenol sebagai Carrier bagi Recovery Logam Berat dengan Teknik Membran Cair. Reaktor, 13(1), pp.16-23.
DOI: 10.14710/reaktor.13.1.16-23
Google Scholar
[24]
Gasser, M.S., El-Hefny, N.E. and Daoud, J.A., 2008. Extraction of Co (II) from aqueous solution using emulsion liquid membrane. Journal of hazardous materials, 151(2-3), pp.610-615.
DOI: 10.1016/j.jhazmat.2007.06.032
Google Scholar