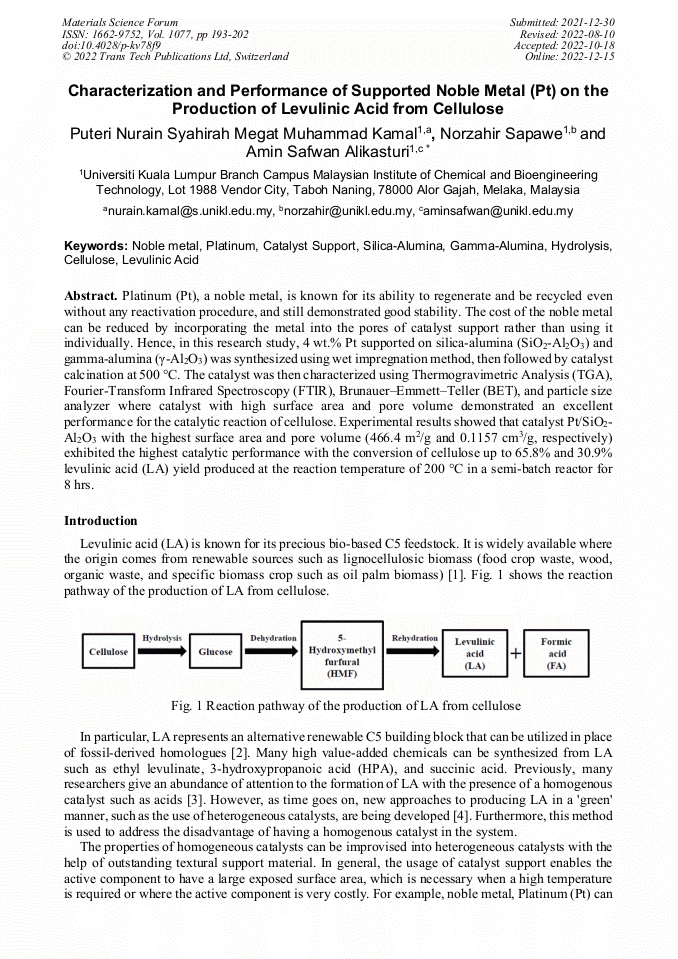
[1]
M. Signoretto, S. Taghavi, E. Ghedini and F. Menegazzo, Catalytic production of levulinic acid (LA) from actual biomass, Molecules (Basel, Switzerland). 24 (2019) 1–20.
DOI: 10.3390/molecules24152760
Google Scholar
[2]
A. Caretto and A. Perosa, Upgrading of levulinic acid with dimethylcarbonate as solvent/ reagent, ACS Sustain. Chem. Eng. 1 (2013), 6–11.
DOI: 10.1021/sc400067s
Google Scholar
[3]
Information on https://openscholar.dut.ac.za/bitstream/10321/1713/1/MTHEMBU_2016.pdf.
Google Scholar
[4]
L. Peng, L. Lin, J. Zhang, J. Zhuang, B. Zhang and Y. Gong, Catalytic conversion of cellulose to levulinic acid by metal chlorides, Molecules. 15 (2010) 5258–5272.
DOI: 10.3390/molecules15085258
Google Scholar
[5]
K. Wang, J. Ye, M. Zhou, P. Liu, X. Liang, J. Xu and J. Jiang, Selective conversion of cellulose to levulinic acid and furfural in sulfolane/water solvent, Cellul. 24 (2017) 1383–1394.
DOI: 10.1007/s10570-016-1184-7
Google Scholar
[6]
G. Busca, Silica-alumina catalytic materials: a critical review, Catal. Today. 357 (2019) 621–629.
DOI: 10.1016/j.cattod.2019.05.011
Google Scholar
[7]
E. J. M. Hensen, D. G. Poduval, P. C. M. M. Magusin, A, E. Coumans and J. A. R. Veen, Formation of acid sites in amorphous silica-alumina, J. Catal. 269 (2010) 201–218.
DOI: 10.1016/j.jcat.2009.11.008
Google Scholar
[8]
M. E. Ali, M. M. Rahman, S. M. Sarkar and S. B. A. Hamid, Heterogeneous metal catalysts for oxidation reactions. J. of Nanomater. 2014 (2014) 1-23.
Google Scholar
[9]
K. Y. Paranjpe, Alpha, beta and gamma alumina as a catalyst - a review, J. Pharm. Innov. 6 (2017) 236–238.
Google Scholar
[10]
P. Kim, Y. Kim, H. Kim, I. K. Song and J. Yi, Synthesis and characterization of mesoporous alumina for use as a catalyst support in the hydrodechlorination of 1,2-dichloropropane: Effect of preparation condition of mesoporous alumina, J. Mol. Catal. A Chem. 219 (2014) 87–95.
DOI: 10.1016/j.molcata.2004.04.038
Google Scholar
[11]
M. A. Usman, T. O. Alaje, V. I. Ekwueme and E. A. Awe, Investigation of the catalytic performance of a novel nickel/kit-6 nanocatalyst for the hydrogenation of vegetable oil, ISRN Chem. Eng. 2012 (2012) 1–6.
DOI: 10.5402/2012/526852
Google Scholar
[12]
D. Ding, J. Wang, J. Xi, X. Liu, G. Lu and Y. Wang, High-yield production of levulinic acid from cellulose and its upgrading to γ- valerolactone, Green Chem. 16 (2014) 3846−3853.
DOI: 10.1039/c4gc00737a
Google Scholar
[13]
K. Wang, Y. Liu, W. Wu, Y. Chen, L. Fang, W. Li and H. Ji, Production of levulinic acid via cellulose conversion over metal oxide-loaded MOF catalysts in aqueous medium, Catal. Letters. 150 (2020) 322–331.
DOI: 10.1007/s10562-019-03023-y
Google Scholar
[14]
A. Shrotri, A. Tanksale, J. N. Beltramini, H. Gurav and S. V. Chilukuri, Conversion of cellulose to polyols over promoted nickel catalysts, Catal. Sci. Techno. 2 (2012) 1852–1858.
DOI: 10.1039/c2cy20119d
Google Scholar
[15]
X. Zhang, L. J. Durndell, M. A. Isaacs, C. M. A. Parlett, A. F. Lee and K. Wilson, Platinum-catalyzed aqueous-phase hydrogenation of d‑glucose to d‑sorbitol, ACS Catal. 6 (2016) 7409–7417.
DOI: 10.1021/acscatal.6b02369
Google Scholar
[16]
P. N. S. M. M. Kamal, N. I. Mohamad, M. E. A. Serit, N.S.A. Rahim, N. I. Jimat, and A. S. Alikasturi, Study on the effect of reaction and calcination temperature towards glucose hydrolysis using solid acid catalyst, Mater. Today: Proc. 31 (2020) 282-286.
DOI: 10.1016/j.matpr.2020.06.008
Google Scholar
[17]
G. Li, W. Liu, C. Ye, X. Li and C. L, Chemocatalytic conversion of cellulose into key platform chemicals, Int. J. Polym. Sci. 2018 (2018) 1-20.
Google Scholar
[18]
M. N. N. Shahirah, J. Gimbun, S. S. Lam, Y. H. Ng, & C. K. Cheng, Synthesis and characterization of a La–Ni/Α-Al2O3 catalyst and its use in pyrolysis of glycerol to syngas, Renew. Energy. 132 (2019) 1389–1401.
DOI: 10.1016/j.renene.2018.09.033
Google Scholar
[19]
M. N. I. N. Azan, P. N. S. M. M. Kamal, M. A. A. Rasmadi, M. H. Adzhar, A. S. A. Taufek, N. S. M. Nasir and A. S. Alikasturi (2020). Production of biodiesel from palm oil refinery pilot plant waste using Ni/CaO (ES) catalyst. Mater. Today: Proc. 31 (2020), 292-299.
DOI: 10.1016/j.matpr.2020.06.012
Google Scholar
[20]
S. S. Joshi, A. D. Zodge, K. V. Pandare, and B. D. Kulkarni, Efficient conversion of cellulose to levulinic acid by hydrothermal treatment using zirconium dioxide as a recyclable solid acid catalyst, Ind. and Eng. Chem. Res. 53 (2014) 18796–18805.
DOI: 10.1021/ie5011838
Google Scholar
[21]
J. E. Mudd, T. J. Gardner and A. G. Sault Platinum Catalyzed Decomposition of Activated Carbon: 1. Initial Studies (2001).
DOI: 10.2172/791893
Google Scholar
[22]
M. Yabushita, H. Kobayashi, K. Hara, and A. Fukuoka, Quantitative evaluation of ball-milling effects on the hydrolysis of cellulose catalysed by activated carbon, Catal. Sci. Techno. 4 (2014) 2312–2317.
DOI: 10.1039/c4cy00175c
Google Scholar
[23]
V. G. Deshmane, S. L. Owen, R. Y. Abrokwah and D. Kuila, Mesoporous nanocrystalline TiO2 supported metal (Cu, Co, Ni, Pd, Zn, and Sn) catalysts: Effect of metal-support interactions on steam reforming of methanol, J. of Mol. Catal. A Chem. 408 (2015) 202–213.
DOI: 10.1016/j.molcata.2015.07.023
Google Scholar
[24]
H. Zhang, H. Huang, Y. Ji, Z. Qiao, C. Zhao and J. He, Preparation, characterization, and application of magnetic Fe-SBA-15 mesoporous silica molecular sieves, J. Auto. Methods and Manage. Chem. 2010 (2010) 323509.
DOI: 10.1155/2010/323509
Google Scholar
[25]
O. Amadine, Y. Essamlali, A. Fihri, M. Larzek, and M. Zahouily, Effect of calcination temperature on the structure and catalytic performance of copper-ceria mixed oxide catalysts in phenol hydroxylation, RSC Adv. 7 (2017) 12586–12597.
DOI: 10.1039/c7ra00734e
Google Scholar
[26]
I. E. Anderson, R. A. Shircliff, C. MacAuley, D. K. Smith, B. G. Lee, S. Agarwal, P. Stradins and R. T. Collins, J. Phys. Chem, 116 (2012) 3979–3987.
DOI: 10.1021/jp211569a
Google Scholar
[27]
K. A. Shah, J. K. Parikh, B. Z. Dholakiya and K. C. Maheria, Fatty acid methyl ester production from acid oil using silica sulfuric acid: process optimization and reaction kinetics, Chem. Pap, 68 (2014) 472–483.
DOI: 10.2478/s11696-013-0488-4
Google Scholar
[28]
A. Gaber, M. A. Abdel- Rahim, A. Y. Abdel-Latief, and M. N. Abdel-Salam, Influence of calcination temperature on the structure and porosity of nanocrystalline SnO2 synthesized by a conventional precipitation method, Int. J. Electrochem. Sci. 9 (2014) 81–95.
DOI: 10.24297/jac.v12i11.4831
Google Scholar
[29]
M. Rahmati, B. Huang, M. K. Mortensen, K. Keyvanloo, T. H. Fletcher, B. F. Woodfield, W. C. Hecker and M. D. Argyle, Effect of different alumina supports on performance of cobalt Fischer-Tropsch catalysts, J. Catal. 359 (2018) 92–100.
DOI: 10.1016/j.jcat.2017.12.022
Google Scholar
[30]
W. Zhang, C. Li, Z. Ma, L. Yang and H. He, Photocatalyst prepared using sol-gel method, J. Adv. Oxid. Technol. 19 (2016) 119–124.
Google Scholar
[31]
S. Pourjafar, J. Kreft, H. Bilek, E. Kozliak and W. Seames, Exploring large pore size alumina and silica-alumina based catalysts for decomposition of lignin, AIMS Energy. 6 (2018) 993–1008.
DOI: 10.3934/energy.2018.6.993
Google Scholar
[32]
Y. Zhang, Y. Tao, J. Huang and P. Williams, Influence of silica–alumina support ratio on H2 production and catalyst carbon deposition from the Ni-catalytic pyrolysis/reforming of waste tyres, Waste Manag. and Res. 35 (2017) 1045–1054.
DOI: 10.1177/0734242x17722207
Google Scholar
[33]
B. M. Weckhuysen and I. E. Wachs, Catalysis by supported metal oxides. Handbook of Surfaces and Interfaces of Materials, Academic Press, 2001, p.613–648.
DOI: 10.1016/b978-012513910-6/50018-9
Google Scholar
[34]
Information on http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-85216.
Google Scholar