p.78
p.85
p.93
p.98
p.106
p.113
p.121
p.128
p.134
Analysis of the Possibility of Use Lithium - Ion as a Starting Battery on the Ship Engine Room
Abstract:
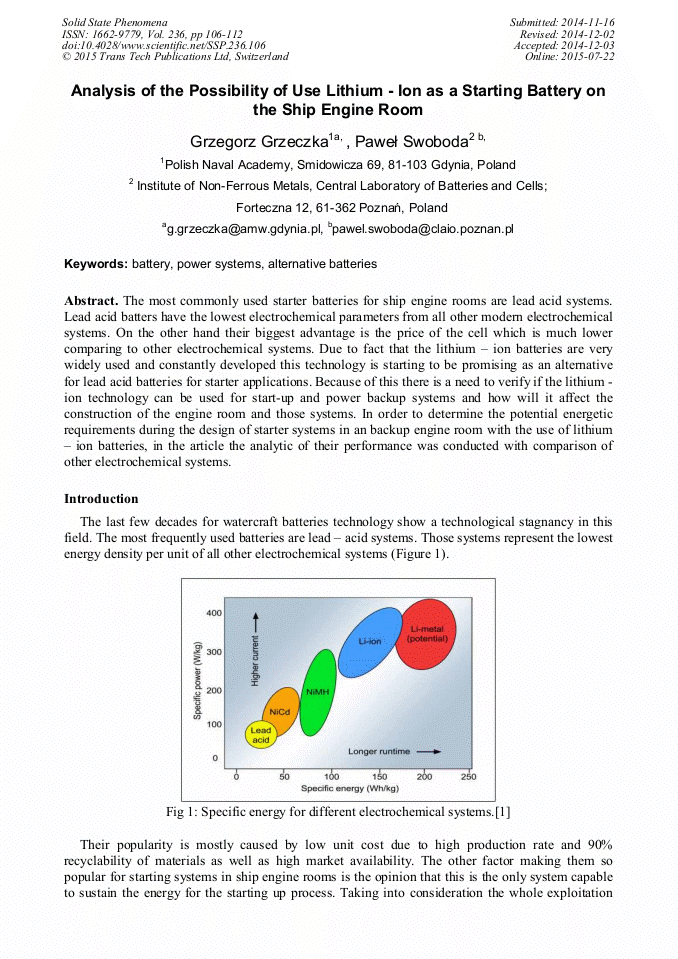
The most commonly used starter batteries for ship engine rooms are lead acid systems. Lead acid batters have the lowest electrochemical parameters from all other modern electrochemical systems. On the other hand their biggest advantage is the price of the cell which is much lower comparing to other electrochemical systems. Due to fact that the lithium – ion batteries are very widely used and constantly developed this technology is starting to be promising as an alternative for lead acid batteries for starter applications. Because of this there is a need to verify if the lithium - ion technology can be used for start-up and power backup systems and how will it affect the construction of the engine room and those systems. In order to determine the potential energetic requirements during the design of starter systems in an backup engine room with the use of lithium – ion batteries, in the article the analytic of their performance was conducted with comparison of other electrochemical systems.
Info:
Periodical:
Pages:
106-112
DOI:
Citation:
Online since:
July 2015
Authors:
Keywords:
Price:
Сopyright:
© 2015 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: