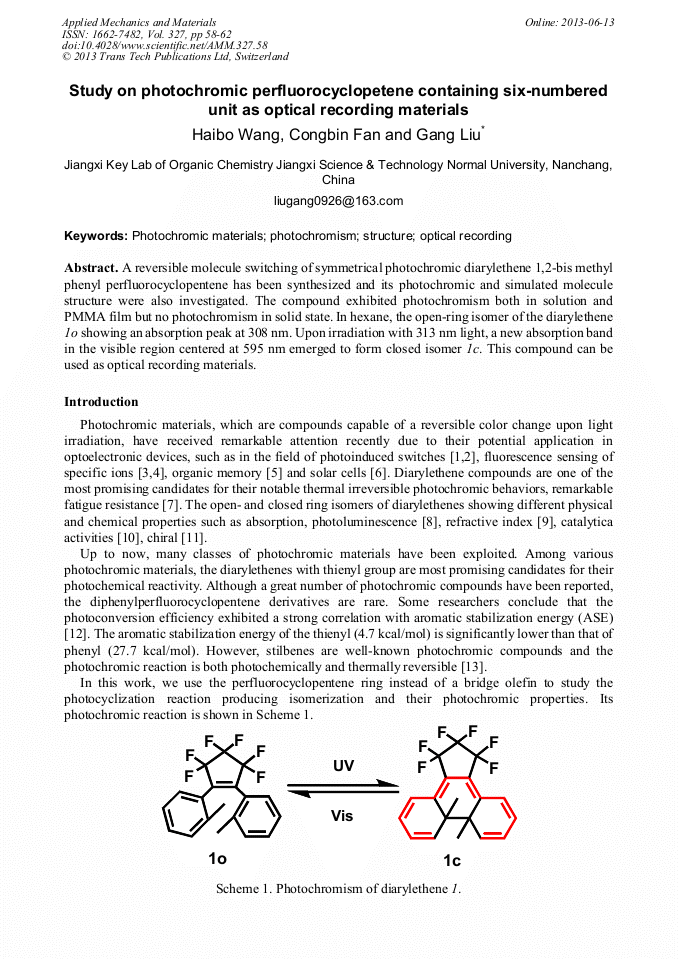
[1]
H. Tian, Y.L. Feng, J. Mater. Chem. vol. 18 (2008) p.1617
Google Scholar
[2]
A.C. Whalley, M.L. Steigerwald, X. Guo, C. Nuckolls, J. Am. Chem. Soc. vol 129 (2007), p.12590
Google Scholar
[3]
Q. Zou, X. Li, J. J. Zhang, J. Zhou, B. B. Sun, and H. Tian, Chem. Commun., vol 48 (2012), p (2095)
Google Scholar
[4]
S. Z. Pu, D. H. Jiang, W. J. Liu, G. Liu, and S. Q. Cui, J. Mater. Chem., vol 22 (2012), p.3517
Google Scholar
[5]
T. Tsujioka, T. Sasa, Y. Kakihara, Org. Electr., vol. 13 (2012), p.681
Google Scholar
[6]
C. B. Fan, P. Yang, X. M. Wang, G. Liu, X. X. Jiang, H. Z. Chen, X. T. Tao, M. Wang, M. H. Jiang, Sol. Energ. Mat. Sol. C., vol. 95 (2011), p.992
Google Scholar
[7]
H. Tian, S. J. Yang, Chem. Soc. Rev. vol 33 (2004), p.85
Google Scholar
[8]
T. Fukaminato, T. Sasaki, T. Kawai, N. Tamai, and M. Irie, J. Am. Chem. Soc. vol 126 (2004), p.14843
Google Scholar
[9]
C. Bertarelli, A. Bianco, Franco D'Amore, M. C. Gallazzi, and G. Zerbi, Adv. Funct. Mater. vol 14 (2004), p.357
Google Scholar
[10]
B. M. Neilson and C. W. Bielawski, J. Am. Chem. Soc. vol 134 (2012), p.12693
Google Scholar
[11]
Y. Li and Q. Li, Org. Lett., vol 14 (2012), p.4362
Google Scholar
[12]
G. Liu, S. Pu, and R. Wang, Org. Lett., vol 15 (2013), p.980
Google Scholar
[13]
K A. Muszkat, Top Curr Chem, vol 88 (1980), p.89
Google Scholar
[14]
Woodward RB, Hoffmann R. The conservation of orbital symmetry.Weinheim, Germany: Verlag Chemie; GmbH, (1970)
Google Scholar
[15]
M. Morimoto, M. Irie, Chem. Eur. J. vol 12 (2006), p.4275
Google Scholar
[16]
V. Ramamurthy, K. Venkatesan, Chem. Rev. vol 87 (1987), p.433
Google Scholar
[17]
Z. X. Li, L. Y. Liao, W. Sun, C. H. Xu, C. Zhang, C. J. Fang, C. H. Yan, J. Phys. Chem. C, vol 112 (2008), p.5190
Google Scholar
[18]
L. Ordronneau, H. Nitadori, I. Ledoux, A. Singh, J.A.G. Williams, M. Akita, V. Guerchais, and H. L. Bozec, Inorg. Chem., vol 51 (2012), p.5627
DOI: 10.1021/ic2025457
Google Scholar
[19]
S.Z Pu, T.S. Yang, J.K. Xu, B. Chen. Tetrahedron Lett. vol 47 (2006), p.6473
Google Scholar
[20]
S.Z Pu, T.S. Yang, B.l. Yao, Y.l. Wang, M. Lei, J.k. Xu. Mater. Lett. vol 61 (2007), p.855
Google Scholar
[21]
S.Z. Pu, C.B. Fan, W.J. Miao, G. Liu. Dyes Pigm. vol 84 (2010), p.25
Google Scholar
[22]
K. Kasatani, S. Kambe, M. Irie, J. Photochem. Photobiol. A: Chem. vol 122 (1999), P 11
Google Scholar