p.22
p.26
p.32
p.40
p.45
p.50
p.57
p.63
p.68
Adsorption Kinetics of Pb2+ Ions Using Chitosan Nanoparticles
Abstract:
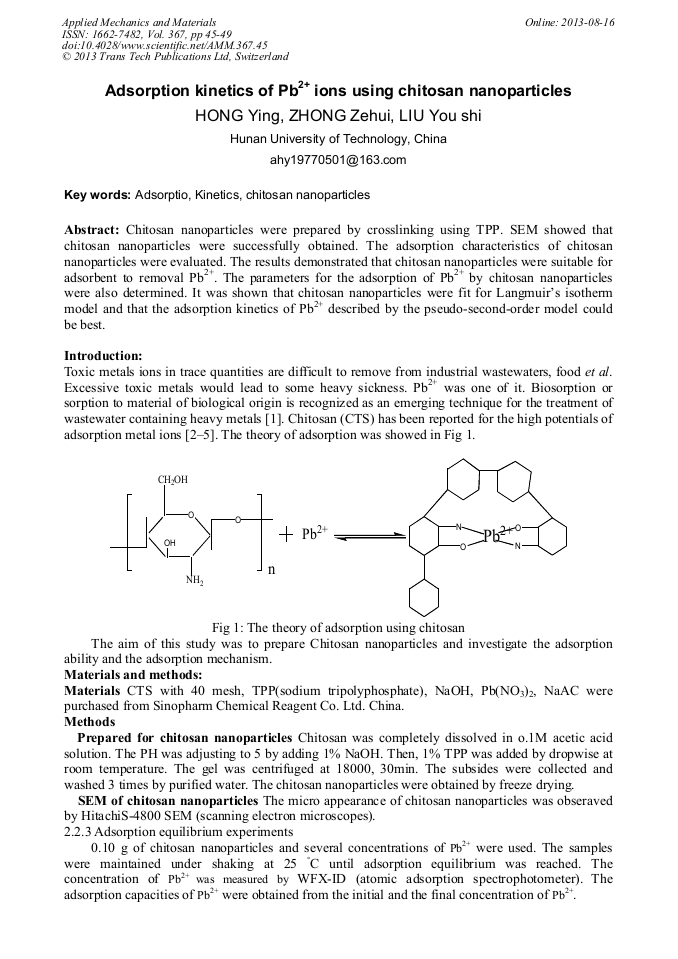
Chitosan nanoparticles were prepared by crosslinkingusing TPP. SEM showed that chitosan nanoparticles were successfully obtained.The adsorption characteristics of chitosan nanoparticles were evaluated. Theresults demonstrated that chitosan nanoparticles were suitable for adsorbent toremoval Pb2+. The parameters for the adsorption of Pb2+by chitosan nanoparticles were also determined. It was shown that chitosannanoparticles were fit for Langmuir’s isotherm model and that the adsorptionkinetics of Pb2+ described by the pseudo-second-order model could bebest.
Info:
Periodical:
Pages:
45-49
DOI:
Citation:
Online since:
August 2013
Authors:
Keywords:
Price:
Сopyright:
© 2013 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: