p.727
p.731
p.735
p.739
p.743
p.748
p.755
p.761
p.767
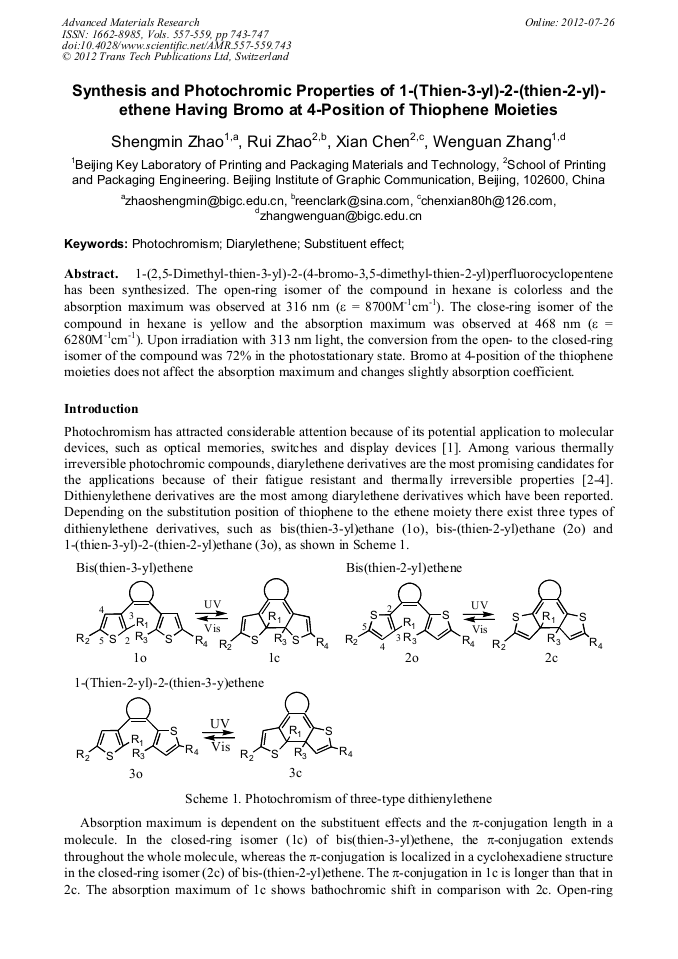
Synthesis and Photochromic Properties of 1-(Thien-3-yl)-2-(thien-2-yl)ethene Having Bromo at 4-Position of Thiophene Moieties
Abstract:
1-(2,5-Dimethyl-thien-3-yl)-2-(4-bromo-3,5-dimethyl-thien-2-yl)perfluorocyclopentene has been synthesized. The open-ring isomer of the compound in hexane is colorless and the absorption maximum was observed at 316 nm (ε = 8700M-1cm-1). The close-ring isomer of the compound in hexane is yellow and the absorption maximum was observed at 468 nm (ε = 6280M-1cm-1). Upon irradiation with 313 nm light, the conversion from the open- to the closed-ring isomer of the compound εwas 72% in the photostationary state. Bromo at 4-position of the thiophene moieties does not affect the absorption maximum and changes slightly absorption coefficient.
Info:
Periodical:
Pages:
743-747
Citation:
Online since:
July 2012
Authors:
Keywords:
Price:
Сopyright:
© 2012 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: