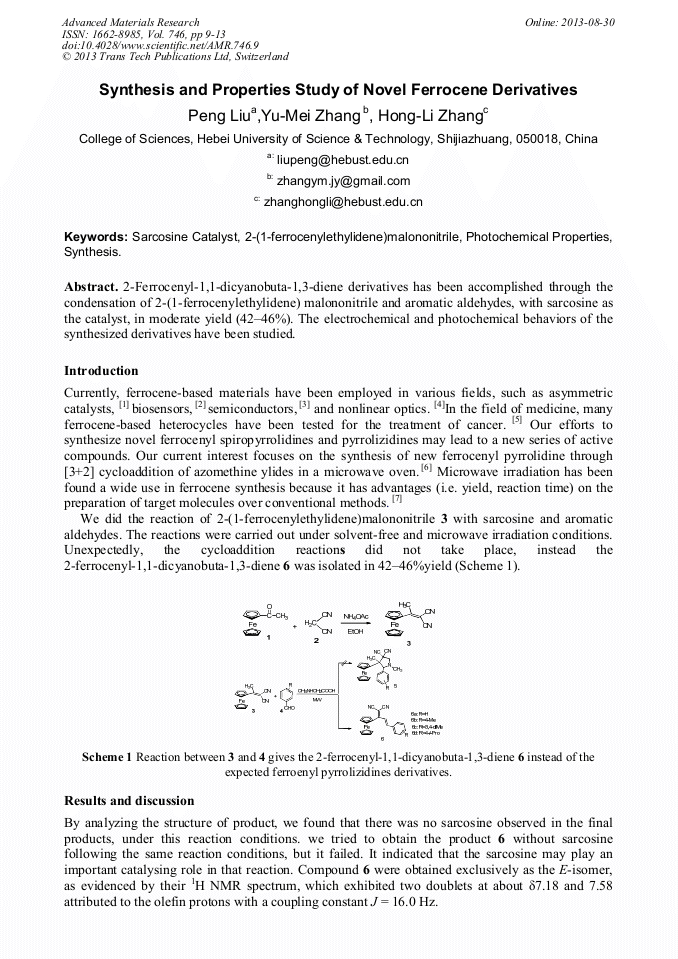
[1]
a) TJ. Zhu, QS. Wu,; P. Chen and YP. Ding, J. Organomet. Chem., 694(2009) 21-26. b) L. Diab, M. Gouygou, E. Manoury, P. Kalck, and M. Urrutigoïty, Tetrahedron Letters, 49(2008) 5186-5189.
DOI: 10.1016/j.tetlet.2008.06.057
Google Scholar
[2]
a) D. Basak and B. Mallik, Synthetic Metals, 156(2006).
Google Scholar
[3]
a) M. Muraoka, S. L. Gillett and T. W. Bell, Angew. Chem. Int. Ed. 41(2002) 3653-3656. b) A. R. Pike, L. C. Ryder, B. R. Horrocks, W. Clegg, B. A. Connoll and A. Houlton, Chem. Eur. J. 11(2005) 344 – 353.
Google Scholar
[4]
a) E. Xenogiannopoulou, M. Medved, K. Iliopoulos, S. Couris, M. G. Papadopoulos, D. Bonifazi, C. Sooambar, A. Mateo-Alonso and M. Prato, ChemPhysChem, 8(2007).
DOI: 10.1002/cphc.200600760
Google Scholar
[5]
a) A. I. Mufula, B. A. Aderibigbe, E. W. Neuse and H. E. Mukaya, J. Inorg. Organomet. P., 22(2012).
Google Scholar
[6]
YM. Zhang, P. Liu, HL. Zhang, GL. Feng and LJ. Geng, Microwave-assisted synthesis of novel ferrocenyl pyrrolizidines,J. Chem. Res. 9(2012) 536-538.
Google Scholar
[7]
a) S. Funda Ekti and Hür Deniz, Inorg. Chem. Communic. 11(2008) 1027-1029. b). H. Deniz, S. Funda Ekti Dal, G. Ali Varol and Evrim Hür, J. Organomet. Chem. 696(2011) 2543-2548.
DOI: 10.1016/j.jorganchem.2011.03.037
Google Scholar
[8]
X-S. Wang, M-M. Zhang, Q. Li and C-S. Yao, Synthetic Commun. 39(2009) 3045-3059.
Google Scholar
[9]
P. D. Jarowski, Y-L. Wu, C. Boudon, J-P. Gisselbrecht, M. Gross, W. B. Schweizer and F. Diederich, Org. Biomol. Chem., 7(2009) 1312-1322.
DOI: 10.1039/b821230a
Google Scholar
[10]
a) S. Venkatraman, R. Kumar, J. Sankar, T. K. Chandrashekar, K. Sendhil, C. Vijayan, A. Kelling, M. O. Senge, Chem. Eur J., 10(2004)1423-1432. b) B. Fabre, Accounts Chem. Res., 43(2010) 1509-1518.
DOI: 10.1002/chem.200305558
Google Scholar