p.262
p.266
p.270
p.274
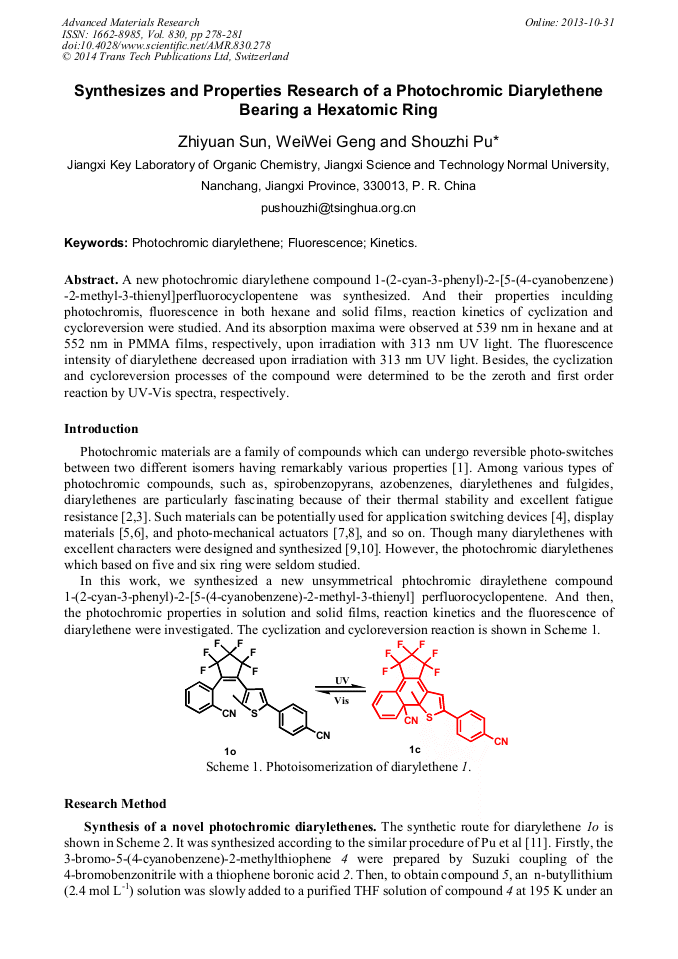
p.278
p.282
p.286
p.290
p.294
Synthesizes and Properties Research of a Photochromic Diarylethene Bearing a Hexatomic Ring
Abstract:
A new photochromic diarylethene compound 1-(2-cyan-3-phenyl)-2-[5-(4-cyanobenzene) -2-methyl-3-thienyl] perfluorocyclopentene was synthesized. And their properties inculding photochromis, fluorescence in both hexane and solid films, reaction kinetics of cyclization and cycloreversion were studied. And its absorption maxima were observed at 539 nm in hexane and at 552 nm in PMMA films, respectively, upon irradiation with 313 nm UV light. The fluorescence intensity of diarylethene decreased upon irradiation with 313 nm UV light. Besides, the cyclization and cycloreversion processes of the compound were determined to be the zeroth and first order reaction by UV-Vis spectra, respectively.
Info:
Periodical:
Pages:
278-281
DOI:
Citation:
Online since:
October 2013
Authors:
Keywords:
Price:
Сopyright:
© 2014 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: