p.81
p.85
p.89
p.93
p.97
p.101
p.106
p.112
p.116
Comparative Study of Stability and Detonation Characterization of AMF and ACF
Abstract:
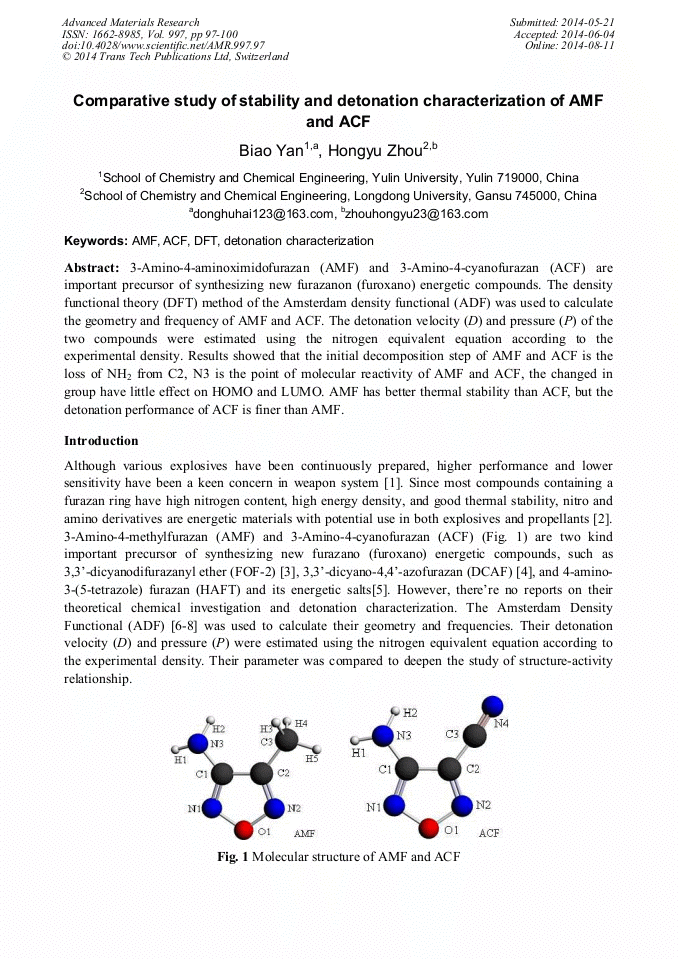
3-Amino-4-aminoximidofurazan (AMF) and 3-Amino-4-cyanofurazan (ACF) are important precursor of synthesizing new furazanon (furoxano) energetic compounds. The density functional theory (DFT) method of the Amsterdam density functional (ADF) was used to calculate the geometry and frequency of AMF and ACF. The detonation velocity (D) and pressure (P) of the two compounds were estimated using the nitrogen equivalent equation according to the experimental density. Results showed that the initial decomposition step of AMF and ACF is the loss of NH2 from C2, N3 is the point of molecular reactivity of AMF and ACF, the changed in group have little effect on HOMO and LUMO. AMF has better thermal stability than ACF, but the detonation performance of ACF is finer than AMF.
Info:
Periodical:
Pages:
97-100
DOI:
Citation:
Online since:
August 2014
Authors:
Keywords:
Price:
Сopyright:
© 2014 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: