p.1293
p.1299
p.1309
p.1316
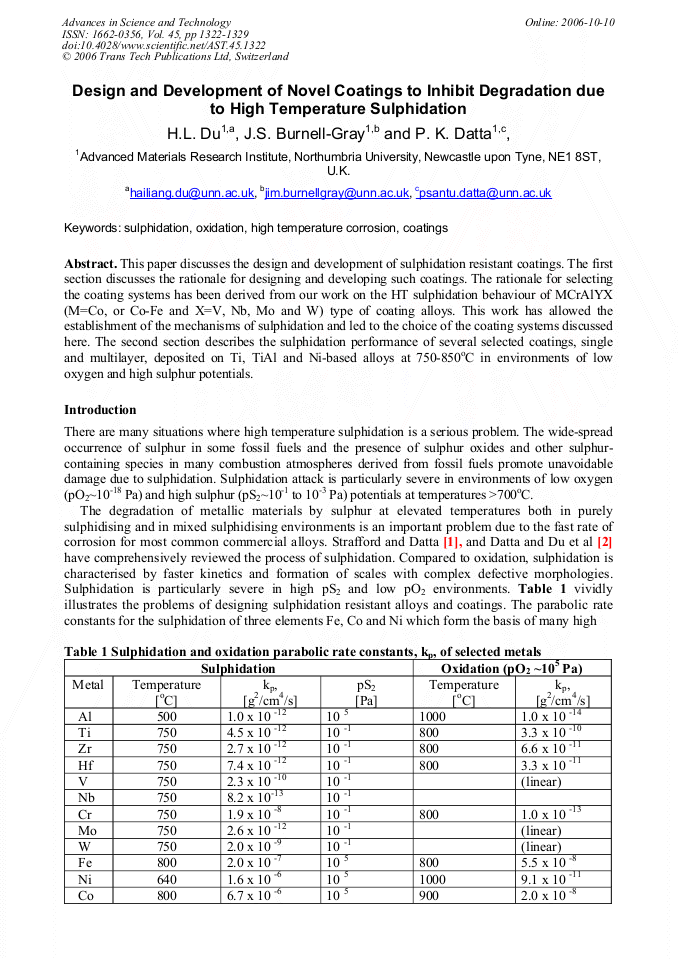
p.1322
p.1330
p.1336
p.1342
p.1351
Design and Development of Novel Coatings to Inhibit Degradation due to High Temperature Sulphidation
Abstract:
This paper discusses the design and development of sulphidation resistant coatings. The first section discusses the rationale for designing and developing such coatings. The rationale for selecting the coating systems has been derived from our work on the HT sulphidation behaviour of MCrAlYX (M=Co, or Co-Fe and X=V, Nb, Mo and W) type of coating alloys. This work has allowed the establishment of the mechanisms of sulphidation and led to the choice of the coating systems discussed here. The second section describes the sulphidation performance of several selected coatings, single and multilayer, deposited on Ti, TiAl and Ni-based alloys at 750-850oC in environments of low oxygen and high sulphur potentials.
Info:
Periodical:
Pages:
1322-1329
DOI:
Citation:
Online since:
October 2006
Authors:
Keywords:
Price:
Сopyright:
© 2006 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: