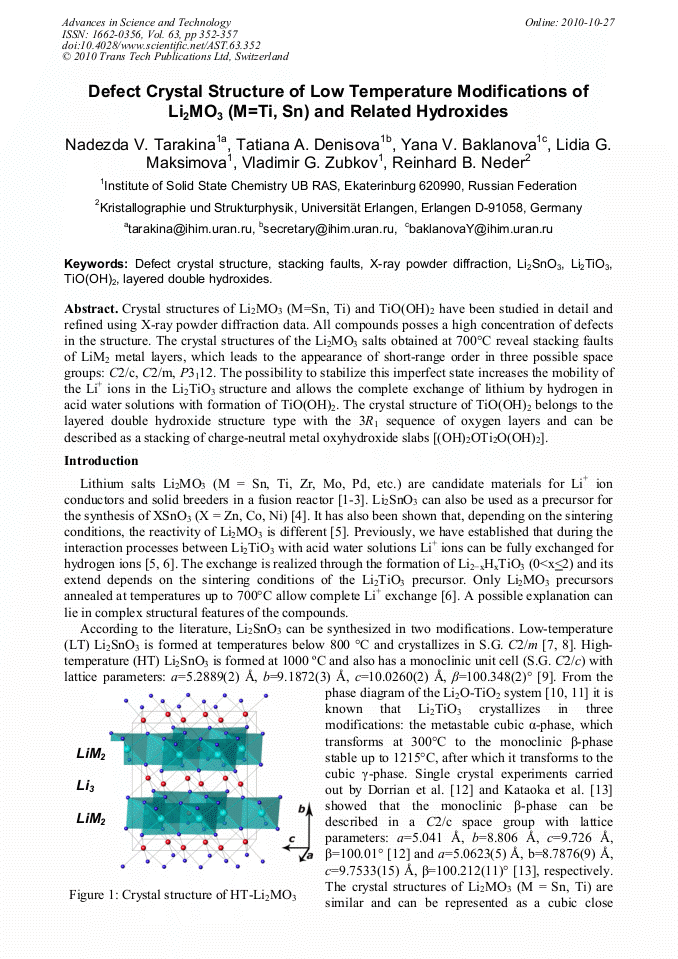
[1]
J.M. Miller, H.B. Hamilton and J.D. Sullevan: J. Nuclear Mat. Vol. 215 (1994), p.877.
Google Scholar
[2]
C. Alvani, S. Casadio, V. Contini, A. Di Bartolomeo, J.D. Lulewicz and N. Roux: J. Nucl. Mater. Vol. 307-311 (2002), p.837.
DOI: 10.1016/s0022-3115(02)01119-4
Google Scholar
[3]
T. Kinjyo, M. Nishikawa, M. Enoeda and S. Fukada: Fusion Eng. Des. Vol. 83 (2008), p.580.
Google Scholar
[4]
D. Kovacheva and K. Petrov: Solid State Ionics Vol. 109 (1998), p.327.
Google Scholar
[5]
T.A. Denisova: International Scientific Journal for Alternative Energy and Ecology, Vol. 3 (2007), p.39.
Google Scholar
[6]
T.A. Denisova, L.G. Maksimova, E.V. Polyakov, N.A. Zhuravlev, S.A. Kovyazina, O.N. Leonidova, D.F. Khabibulin and E.I. Yureva: Russ. J. Inorg. Chem. Vol. 51 (2006), p.691.
DOI: 10.1134/s0036023606050019
Google Scholar
[7]
G.V. Lang: Z. Anorg. Allg. Chem. Vol. 348 (1966), p.246.
Google Scholar
[8]
Von M. Trömel and J. Hauck: Z. Anorg. Allg. Chem. Vol. 373 (1970), p.8.
Google Scholar
[9]
J.L. Hodeau, M. Marezio, A. Santoro and R.S. Roth: J. Solid State Chemistry Vol. 45 (1982), p.170.
Google Scholar
[10]
G. Izquierdo and A.R. West: Mater. Res. Bull. Vol. 15 (1980), p.1655.
Google Scholar
[11]
C. Gicquel, M. Mayer and R. Bouaziz: Compt. Rend. (Paris) Ser. C, Vol. 275 (1972), p.1427.
Google Scholar
[12]
J.F. Dorrian and R.E. Newnham: Mater. Res. Bull. Vol. 4 (1969), p.179.
Google Scholar
[13]
K. Kataoka, Y. Takahashi, N. Kijima, H. Nagai, J. Akimoto, Y. Idemoto and K. Ohshima: Mater. Res. Bull. Vol. 44 (2009), p.168.
Google Scholar
[14]
T. Hoshino, K. Tanaka, J. Makita and T. Hashimoto: J. Nucl. Mater. Vol. 367-370 (2007), p.1052.
Google Scholar
[15]
D.J.D. Concoran, D.P. Tunstall and J.T.S. Irvine: Solid State Ionics Vol. 136-137 (2000), p.297.
Google Scholar
[16]
A. Le Bail and J.L. Fourquet: Mater. Res. Bull. Vol. 27 (1992), p.75.
Google Scholar
[17]
H. Izawa, S. Kikkawa and M. Koizumi: J. Phys. Chem. Vol. 86 (1982), p.5023.
Google Scholar
[18]
Y.V. Baklanova: Magn. Reson. Solids Vol. 10 (2008), p.39.
Google Scholar
[19]
K. Yawata: Research report of Tsuruoka national college of technology Vol. 41 (2006), p.53.
Google Scholar
[20]
R.B. Neder and T. Proffen: Diffuse scattering and defect structure simulation (Oxford University Press, New York, 2008).
Google Scholar
[21]
A.C. Larson and R.B. Von Dreele: General Structure Analysis System-GSAS (Los Alamos National Laboratory Report LAUR 86-748, 2000).
Google Scholar
[22]
J. Breger, M. Jiang, N. Dupre, Y.S. Meng, Y. Shao-Horn, G. Ceder and C.P. Grey: J. Solid State Chem. Vol. 178 (2005), p.2575.
Google Scholar
[23]
A.V. Radna, C. Shivakumara and P. Vishnu Kamath: Clays Clay Miner. Vol. 53 (2005), p.520.
Google Scholar