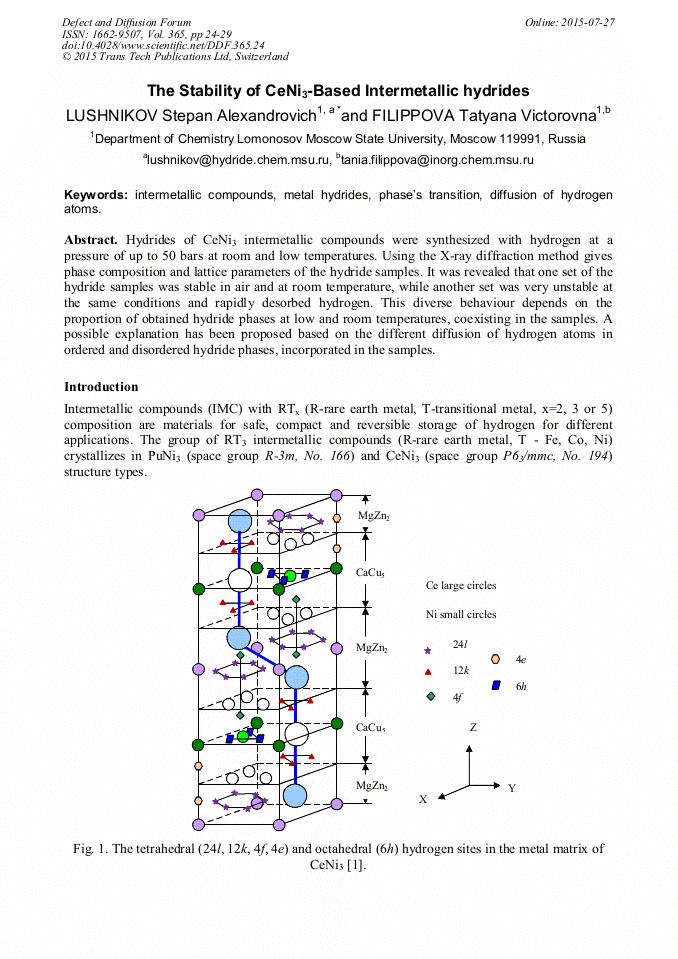
p.1
p.5
p.11
p.17
p.24
p.30
p.36
p.42
p.49
The Stability of CeNi3-Based Intermetallic Hydrides
Abstract:
Hydrides of CeNi3 intermetallic compounds were synthesized with hydrogen at a pressure of up to 50 bars at room and low temperatures. Using the X-ray diffraction method gives phase composition and lattice parameters of the hydride samples. It was revealed that one set of the hydride samples was stable in air and at room temperature, while another set was very unstable at the same conditions and rapidly desorbed hydrogen. This diverse behaviour depends on the proportion of obtained hydride phases at low and room temperatures, coexisting in the samples. A possible explanation has been proposed based on the different diffusion of hydrogen atoms in ordered and disordered hydride phases, incorporated in the samples.
Info:
Periodical:
Pages:
24-29
DOI:
Citation:
Online since:
July 2015
Price:
Сopyright:
© 2015 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: