p.349
p.353
p.358
p.363
p.367
p.371
p.375
p.380
p.384
Oxidation Behavior of Amorphous Nano-Si3N4 Powders
Abstract:
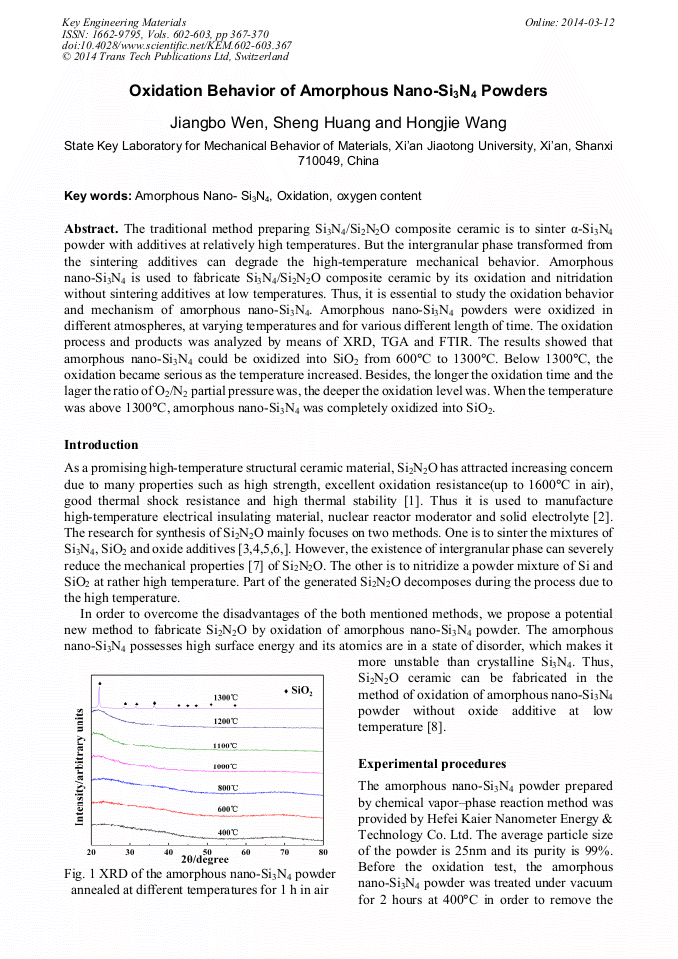
The traditional method preparing Si3N4/Si2N2O composite ceramic is to sinter α-Si3N4 powder with additives at relatively high temperatures. But the intergranular phase transformed from the sintering additives can degrade the high-temperature mechanical behavior. Amorphous nanoSi3N4 is used to fabricate Si3N4/Si2N2O composite ceramic by its oxidation and nitridation without sintering additives at low temperatures. Thus, it is essential to study the oxidation behavior and mechanism of amorphous nanoSi3N4. Amorphous nanoSi3N4 powders were oxidized in different atmospheres, at varying temperatures and for various different length of time. The oxidation process and products was analyzed by means of XRD, TGA and FTIR. The results showed that amorphous nanoSi3N4 could be oxidized into SiO2 from 600°C to 1300°C. Below 1300°C, the oxidation became serious as the temperature increased. Besides, the longer the oxidation time and the lager the ratio of O2/N2 partial pressure was, the deeper the oxidation level was. When the temperature was above 1300°C, amorphous nanoSi3N4 was completely oxidized into SiO2.
Info:
Periodical:
Pages:
367-370
Citation:
Online since:
March 2014
Authors:
Keywords:
Price:
Сopyright:
© 2014 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: