p.175
p.180
p.185
p.190
p.194
p.201
p.206
p.211
p.216
The Effect of Heat Treatment on Fe2+/Fe3+ Ratio in Soda-Lime Silicate Glass
Abstract:
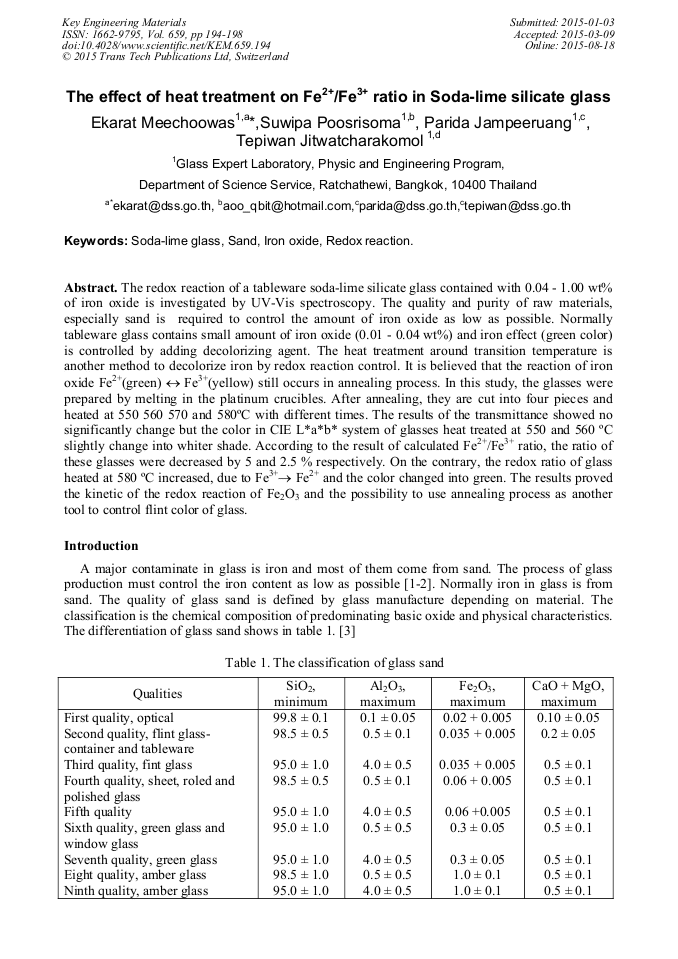
The redox reaction of a tableware soda-lime silicate glass contained with 0.04 - 1.00 wt% of iron oxide is investigated by UV-Vis spectroscopy. The quality and purity of raw materials, especially sand is required to control the amount of iron oxide as low as possible. Normally tableware glass contains small amount of iron oxide (0.01 - 0.04 wt%) and iron effect (green color) is controlled by adding decolorizing agent. The heat treatment around transition temperature is another method to decolorize iron by redox reaction control. It is believed that the reaction of iron oxide Fe2+(green) « Fe3+(yellow) still occurs in annealing process. In this study, the glasses were prepared by melting in the platinum crucibles. After annealing, they are cut into four pieces and heated at 550 560 570 and 580°C with different times. The results of the transmittance showed no significantly change but the color in CIE L*a*b* system of glasses heat treated at 550 and 560 °C slightly change into whiter shade. According to the result of calculated Fe2+/Fe3+ ratio, the ratio of these glasses were decreased by 5 and 2.5 % respectively. On the contrary, the redox ratio of glass heated at 580 °C increased, due to Fe3+to Fe2+ and the color changed into green. The results proved the kinetic of the redox reaction of Fe2O3 and the possibility to use annealing process as another tool to control flint color of glass.
Info:
Periodical:
Pages:
194-198
DOI:
Citation:
Online since:
August 2015
Keywords:
Price:
Сopyright:
© 2015 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: