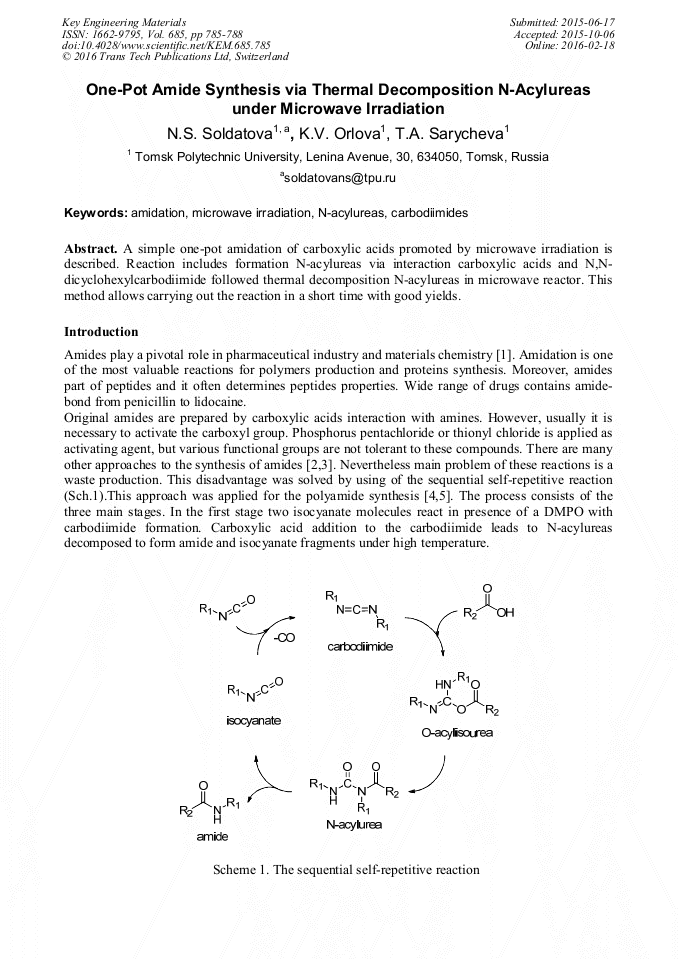
[1]
A. Greenberg, C. M. Breneman, J. F. Liebman The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Material Science, first ed., Wiley, (2000).
Google Scholar
[2]
V. R. Pattabiraman, J. W. Bode Rethinking amide bond synthesis, Nature 480 (2011) 471–479.
DOI: 10.1038/nature10702
Google Scholar
[3]
E. Valeur, M. Bradley Amide bond formation: beyond the myth of coupling reagents, Chem. Soc. Rev., 38 (2009) 606-631.
DOI: 10.1039/b701677h
Google Scholar
[4]
K. -L. Wei, C. -H. Wu, W. -H. Huang, J. -J. Lin, S.A. Dai N-Aryl Acylureas as Intermediates in Sequential Self-Repetitive Reactions To Form Poly(amide−imide)s, Macromolecules, 39 (2006) 12–14.
DOI: 10.1021/ma051827m
Google Scholar
[5]
C. -W. Chen , C. -C. Cheng, S. A. Dai Reactive Macrocyclic Ether−Urethane Carbodiimide (MC−CDI): Synthesis, Reaction, and Ring-Opening Polymerization (ROP), Macromolecules, 40 (2007), 8139–8141.
DOI: 10.1021/ma0713916
Google Scholar
[6]
Schall, Chem. Ber., 26 (1893) 1182.
Google Scholar
[7]
A.H.M. Schotman, W. Mijs, Carbodiimides as important intermediates in the reaction of isocyanates with carboxylic acids J. Trav. Chim. Pays-Bas, 111 (1992) 88–91.
DOI: 10.1002/recl.19921110205
Google Scholar
[8]
A. H. M. Schotman, Mechanism of the reaction of carbodiimides with carboxylic acids J. Trav. Chim. Pays-Bas, 110 (1991) 319-324.
DOI: 10.1002/recl.19911100704
Google Scholar
[9]
K. Kishikawa, H. Eida, S. Kohmoto, M. Yamamoto, K Yamada Chemoselective alcoholysis of acylureas Synthesis, 2 (1994) 173 – 175.
DOI: 10.1055/s-1994-25432
Google Scholar
[10]
J. M. Herbert, A. T. Hewson, J. E. Peace One-Pot Reduction of Carboxylic Acids via O-Acylisoureas, Synth. Commun., 28 (1998) 823-832.
DOI: 10.1080/00032719808006479
Google Scholar
[11]
M. G. Quintanilla, G. Montero, A. G. Hierro, J. M. Diaz, S. Bassani Electrochemical reduction of N-acylureas, Acta Chem. Scand. 53 (1999) 928 – 931.
DOI: 10.3891/acta.chem.scand.53-0928
Google Scholar