p.31
p.38
p.46
p.50
p.55
p.59
p.65
p.75
p.79
Detection of pH Increase at the Surface of Cathodically Polarized Metal by Means of Amphoteric Metals Activation
Abstract:
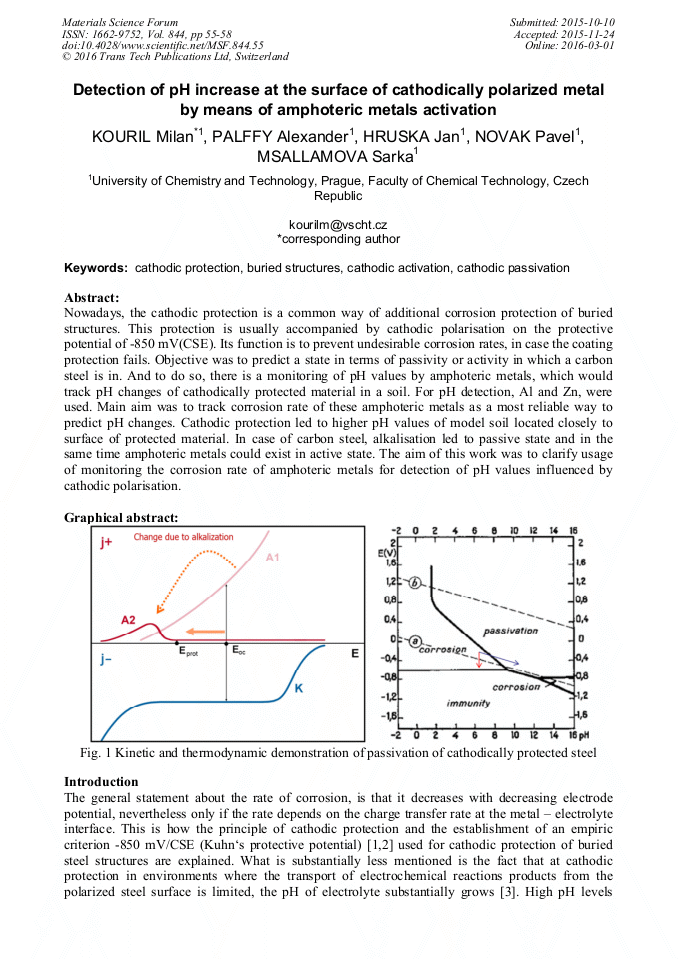
Nowadays, the cathodic protection is a common way of additional corrosion protection of buried structures. This protection is usually accompanied by cathodic polarisation on the protective potential of-850 mV(CSE). Its function is to prevent undesirable corrosion rates, in case the coating protection fails. Objective was to predict a state in terms of passivity or activity in which a carbon steel is in. And to do so, there is a monitoring of pH values by amphoteric metals, which would track pH changes of cathodically protected material in a soil. For pH detection, Al and Zn, were used. Main aim was to track corrosion rate of these amphoteric metals as a most reliable way to predict pH changes. Cathodic protection led to higher pH values of model soil located closely to surface of protected material. In case of carbon steel, alkalisation led to passive state and in the same time amphoteric metals could exist in active state. The aim of this work was to clarify usage of monitoring the corrosion rate of amphoteric metals for detection of pH values influenced by cathodic polarisation.Graphical abstract: Fig. 1 Kinetic and thermodynamic demonstration of passivation of cathodically protected steel
Info:
Periodical:
Pages:
55-58
DOI:
Citation:
Online since:
March 2016
Authors:
Price:
Сopyright:
© 2016 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: