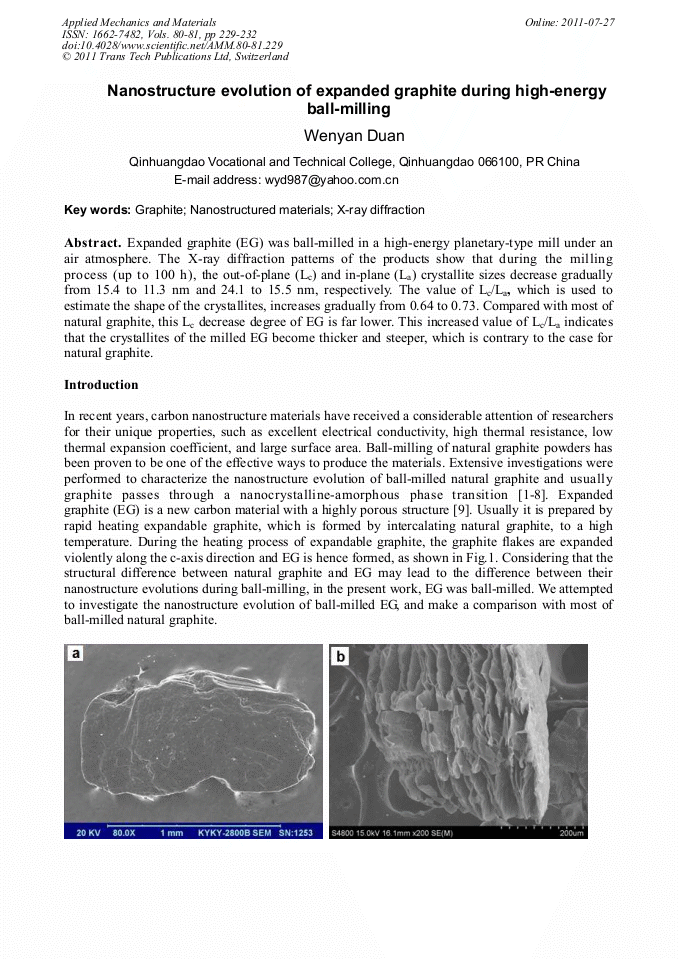
[1]
Y. A. Kim, T. Hayashi, Y. Fukai, M. Endo, T. Yanagisawa, M. S. Dresselhaus, Effect of ball milling on morphology of cup stacked carbon nanotubes, Chem. Phys. Lett. 355 (2002) 279–284.
DOI: 10.1016/s0009-2614(02)00248-8
Google Scholar
[2]
T. Fukunaga, K. Nagano, U. Mizutani, H. Wakayama, Y. Fukushima, Structural change of graphite subjected to mechanical milling, J. Non-Cryst. Solids 232–234 (1998) 416–420.
DOI: 10.1016/s0022-3093(98)00495-5
Google Scholar
[3]
T. D. Shen, W. Q. Ge, K.Y. Wang, M.X. Quan, J.T. Wang, W. D. Wei, et al, Structural disorder and phase transformation in graphite produced by ball-milling, Nanostruct. Mater. 7 (1996) 393–399.
DOI: 10.1016/0965-9773(96)00010-4
Google Scholar
[4]
Y. Chen, J. F. Gerald, L.T. Chadderton, L. Chaffron, Nanoporous carbon produced by ball-milling, Appl. Phys. Lett. 74 (1999) 2782–2784.
DOI: 10.1063/1.124012
Google Scholar
[5]
R. Janot, D. Guerard, Ball-milling: the behavior of graphite as a function of the dispersal media, Carbon 40 (2002) 2887–2896.
DOI: 10.1016/s0008-6223(02)00223-3
Google Scholar
[6]
T.S. Ong, H. Yang, Effect of atmosphere on the mechanical milling of natural graphite, Carbon 38 (2000) 2077–(2085).
DOI: 10.1016/s0008-6223(00)00064-6
Google Scholar
[7]
N. J. Welham, V. Berbenni, P. G. Chapman, Increased chemisorption onto activated carbon after ball-milling, Carbon (2002) 2307–2315.
DOI: 10.1016/s0008-6223(02)00123-9
Google Scholar
[8]
A. Mileva, M. l Wilson, G.S. Kamali Kannangara, N. Tran, X-ray diffraction line profile analysis of nanocrystalline graphite, Mater. Chem. Phys. 111 (2008) 346–350.
DOI: 10.1016/j.matchemphys.2008.04.024
Google Scholar
[9]
M. Inagaki, R. Tashiro, Y. Washino, M. Toyoda, Exfoliation process of graphite via intercalation compounds with sulfuric acid, J. Phys. Chem. Solids 65(2004) 133–137.
DOI: 10.1016/j.jpcs.2003.10.007
Google Scholar
[10]
M. Francke, H. Hermann, R. Wenzel, G. Seifert, K. Wetzig, Modification of carbon nanostructures by high energy ball-milling under argon and hydrogen atmosphere, Carbon 43 (2005) 1204-1212.
DOI: 10.1016/j.carbon.2004.12.013
Google Scholar
[11]
X.H. Chen, H.S. Yang, G.T. Wu, M. Wang, F.M. Deng, et al, Generation of curved or closed-shell carbon nanostructures by ball-milling of graphite, J. Cryst. Growth 218 (2000) 57–61.
DOI: 10.1016/s0022-0248(00)00486-3
Google Scholar
[12]
Y.C. Fan , L.J. Wang , J.L. Li , J. Q. Li , S.K. Sun, F. Chen, L.D. Chen, W. Jiang, Preparation and electrical properties of grapheme nanosheet/Al2O3 composites, carbon 48 (2010)1743-1749.
DOI: 10.1016/j.carbon.2010.01.017
Google Scholar
[13]
R.A. Reynolds III, R.A. Greinke, Influence of expansion volume of intercalated graphite on tensile properties of flexible graphite, Carbon 39 (2001) 473–481.
DOI: 10.1016/s0008-6223(00)00291-8
Google Scholar