p.366
p.371
p.376
p.382
p.387
p.392
p.398
p.403
p.408
Synthesis and Crystal Structure of a New Ni2+ Complex1
Abstract:
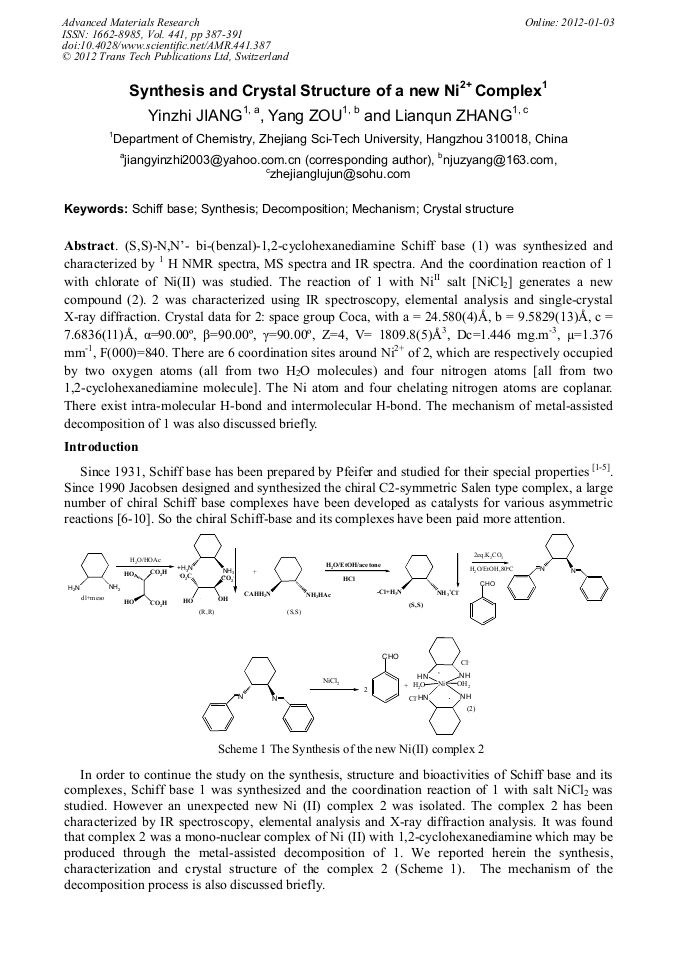
(S,S)-N,N’- bi-(benzal)-1,2-cyclohexanediamine Schiff base (1) was synthesized and characterized by 1 H NMR spectra, MS spectra and IR spectra. And the coordination reaction of 1 with chlorate of Ni(II) was studied. The reaction of 1 with NiII salt [NiCl2] generates a new compound (2). 2 was characterized using IR spectroscopy, elemental analysis and single-crystal X-ray diffraction. Crystal data for 2: space group Coca, with a = 24.580(4)Å, b = 9.5829(13)Å, c = 7.6836(11)Å, α=90.00º, β=90.00º, γ=90.00º, Z=4, V= 1809.8(5)Å3, Dc=1.446 mg.m-3, µ=1.376 mm-1, F(000)=840. There are 6 coordination sites around Ni2+ of 2, which are respectively occupied by two oxygen atoms (all from two H2O molecules) and four nitrogen atoms [all from two 1,2-cyclohexanediamine molecule]. The Ni atom and four chelating nitrogen atoms are coplanar. There exist intra-molecular H-bond and intermolecular H-bond. The mechanism of metal-assisted decomposition of 1 was also discussed briefly.
Info:
Periodical:
Pages:
387-391
DOI:
Citation:
Online since:
January 2012
Authors:
Keywords:
Price:
Сopyright:
© 2012 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: