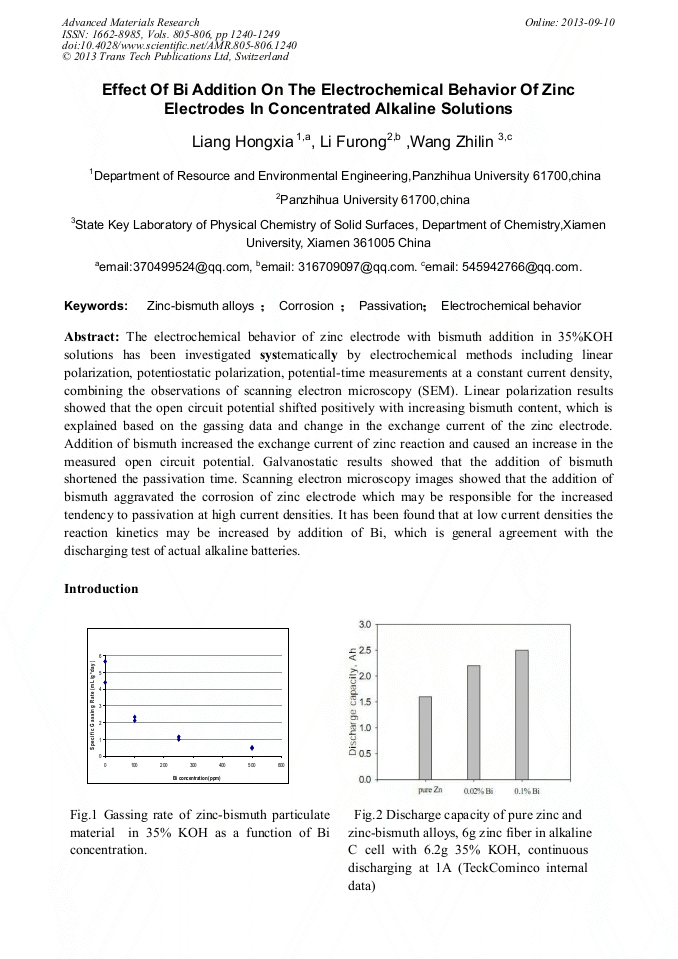
[1]
X.G. Zhang, Corrosion and Electrochemistry of Zinc, Plenum Publishers, New York (1996).
Google Scholar
[2]
X.G. Zhang. J. Power Sources 163 (2006) 591-597.
Google Scholar
[3]
R.M. Dell. Solid State Ionics 134 (2000) 139-158.
Google Scholar
[4]
McLarnon, F. R., and Cairns, E.J.: The secondary alkaline zinc electrode, J. Electrochem. Soc. 138, 645-664, (1991).
DOI: 10.1149/1.2085653
Google Scholar
[5]
McBreen, J., and Cairns, E.J.: The zinc electrode, in Advances in Electrochemistry and Electrochemical Engineering, Vol. 11, H. Gerischer and C. W. Tobias(eds. ), pp.273-352, John Wiley & sons, New York, (1978).
Google Scholar
[6]
Gouda, K. K., Khedr, M. G. A., and Shams EI Din, A. M.: Role of anions in the corrosion and corrosion-inhibition of zinc in aqueous solutions, Corros. Sci. 7, 221-230, (1967).
DOI: 10.1016/s0010-938x(67)80084-2
Google Scholar
[7]
Kordesch, K. V.: Alkaline manganese dioxide zinc batteries, in Batteries, K. V. Kordesch (ed), Vol. 1, pp.241-384, Marcel Dekker, New York, (1974).
DOI: 10.1007/978-1-4615-6687-8_6
Google Scholar
[8]
Brodd, R. J.: Batteries for Cordless Appliances, Research Studies Press, Letchworth, England, (1987).
Google Scholar
[9]
Huber, R.: Leclanche batteries, in Batteries, K. V. Kordesch (ed. ), Vol. 1, pp.1-239, Marcel Dekker, New York, (1974).
Google Scholar
[10]
Lee, T. S.: Hydrogen overpotential on zinc containing small amounts of impurities in concentrated alkaline solution, J. Electrochem. Soc. 120, 707-709, (1973).
DOI: 10.1149/1.2403541
Google Scholar
[11]
Miura, A., Takata, K., Okazaki, R., Ogawa, H., Uemura, T., Nakamura, Y., and Kasahara, N.: Development of corrosion resistant zinc alloys for alkaline-manganese batteries, Denki Kagaku 1989(6), 57, 459.
Google Scholar
[12]
Dirkse, T. P.: The behaviour of the zinc electrode in alkaline solutions, J. Electrochem. Soc. 126, 541-543, (1979).
Google Scholar
[13]
Yoshizawa, H., and Miura, A.: Mercury free alkaline manganese batteries, Prog. Batteries Battery Mater. 12, 132-139, (1993).
Google Scholar
[14]
N. Pistofidis, G. Vourlias, S. Konidaris, El. Pavlidou, A. Stergiou, G. Stergioudis. The effect of bismuth on the structure of zinc hot-dip galvanized coatings. Materials Letters. 61, 994-997, (2007).
DOI: 10.1016/j.matlet.2006.06.029
Google Scholar
[15]
M. Gagne, Bull. Bismuth Inst. 72(1998)1.
Google Scholar
[16]
P. Beguin, M. Bosschaerts, D. Dhaussy, R. Pankert, M. Gilles, Bull. Bismuth Inst. 76(2000)1.
Google Scholar
[17]
O. Akinlade, R. N. Singh, F. Sommer. Thermodynamic investigation of viscosity in Cu-Bi and Bi-Zn liquid alloys. Journal of Alloys and Compounds. 267, 195-198, (1998).
DOI: 10.1016/s0925-8388(97)00547-1
Google Scholar
[18]
Bocchi, N., da Cunha, M. R., and D, Alkaline, C.V.: The passivating films of zinc in alkaline solution, Key Eng. Mater. 20-28, 3941-3946, (1988).
DOI: 10.4028/www.scientific.net/kem.20-28.3941
Google Scholar
[19]
Chang, Y., and Prentice, G.: A model for the anodic dissolution of the zinc electrode in the prepassive region, J. Electrochem. Soc. 136, 3398-3403, (1989).
DOI: 10.1149/1.2096460
Google Scholar
[20]
Dirkse, T. P.: Voltage decay at passivated zinc anodes, J. Appl. Electrochem. 1, 27-33, (1971).
DOI: 10.1007/bf00615743
Google Scholar
[21]
Hull, M. N., Ellison, J. Electrochem. Soc. 117, 192-198, (1970).
Google Scholar
[22]
Sato, Y., Niki, H., and Takamura, T.: Effects of carbonate on the anodic dissolution and the passivation of zinc electrode in concentrated solution of potassium hydroxide, J. Electrochem. Soc. 118, 1269-1272, (1971).
DOI: 10.1149/1.2408303
Google Scholar
[23]
Dirkse, T. P., and Hampson, N. A.: The anodic behaviour of zinc in aqueous KOHsolution. Part Ⅰ. Passivation experiments at very high current densities, Electrochim. Acta 16, 2049-2056, (1971).
DOI: 10.1016/0013-4686(71)85018-1
Google Scholar
[24]
Passivity and passivity breakdown of a zinc electrode in aerated neutral sodium nitrate solutions. Electrochimica Acta 50 (2005) 1265-1274.
DOI: 10.1016/j.electacta.2004.07.051
Google Scholar
[25]
Liu, M., Cook, G. M., and Yao, N.P.: Passivation of zinc anodes in KOH electrolytes, J. Electrochem. Soc. 128, 1663-1668, (1981).
DOI: 10.1149/1.2127707
Google Scholar
[26]
X.G. Zhang, Zinc electrode: Form, morphology and Reactivity, Chapter in Encyclopedia of Power Sources, Elsevier, in preparation.
Google Scholar