p.209
p.213
p.217
p.221
p.225
p.229
p.233
p.237
p.241
Effects of LiF Addition in Lithium Ion Conductor La0.56Li0.33TiO3
Abstract:
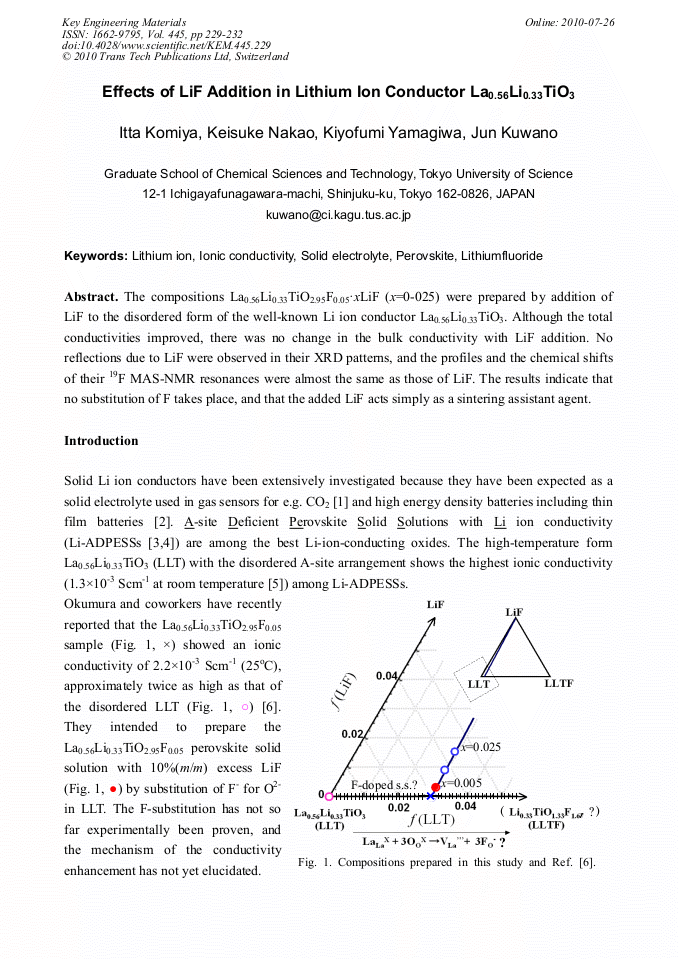
The compositions La0.56Li0.33TiO2.95F0.05•xLiF (x=0-025) were prepared by addition of LiF to the disordered form of the well-known Li ion conductor La0.56Li0.33TiO3. Although the total conductivities improved, there was no change in the bulk conductivity with LiF addition. No reflections due to LiF were observed in their XRD patterns, and the profiles and the chemical shifts of their 19F MAS-NMR resonances were almost the same as those of LiF. The results indicate that no substitution of F takes place, and that the added LiF acts simply as a sintering assistant agent.
Info:
Periodical:
Pages:
229-232
DOI:
Citation:
Online since:
July 2010
Authors:
Keywords:
Price:
Сopyright:
© 2010 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: