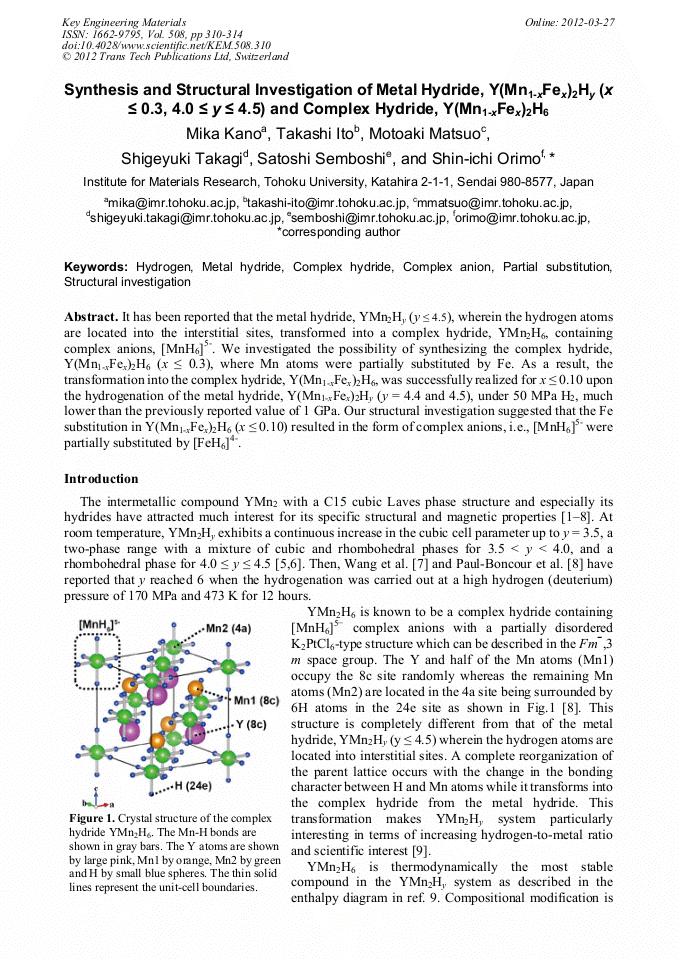
[1]
Y. Nakamura, M. Shiga, S. Kawano, Physica B+C 120 (1983) 212–215.
Google Scholar
[2]
H. Fujii, M. Saga, T. Okamoto, Journal of Less-Common Metals 130 (1987) 25–31.
Google Scholar
[3]
I. N. Goncharenko, I. Mirebeau, A. V. Irodova, E. Suard, Physical Review B 56 (1997) 2580–2584.
Google Scholar
[4]
H. Figiel, J. Przewoznik, V. Paul-Boncour, A. Lindbaum, E. Gratz, M. Latroche, M. Escorne, A. Percheron-Guégan, P. Mietniowski, Journal of Alloys and Compounds 274 (1998) 29–37.
DOI: 10.1016/s0925-8388(98)00566-0
Google Scholar
[5]
J. Przewoznik, V. Paul-Boncour, M. Latroche, A. Percheron-Guégan, Journal of Alloys and Compounds 225 (1995) 436–439.
DOI: 10.1016/0925-8388(94)07048-2
Google Scholar
[6]
M. Latroche, V. Paul-Boncour, A. Percheron-Guégan, F. Bourée-Vigneron, Journal of Alloys and Compounds 274 (1998) 59–64.
DOI: 10.1016/s0925-8388(98)00539-8
Google Scholar
[7]
C. -Y. Wang, V. Paul-Boncour, C. -C. Kang, R. -S. Liu, S. M. Filipek, M. Dorogova, I. Marchuk, T. Hirata, A. Percheron-Guégan, H. -S. Sheu, L. -Y. Jang, J. -M. Chen, H. -D. Yang, Solid State Communications 130 (2004)
815–820.
DOI: 10.1016/j.ssc.2004.03.054
Google Scholar
[8]
V. Paul-Boncour, S. M. Filipek, M. Dorogova, F. Bourée, G. André, I. Marchuk, A. Percheron-Guégan, R. S. Liu, Journal of Solid State Chemistry 178 (2005) 356–362.
DOI: 10.1016/j.jssc.2004.05.066
Google Scholar
[9]
M. Matsuo, K. Miwa, S. Semboshi, H. -W. Li, M. Kano, S. Orimo, Applied Physics Letters 98 (2011) 221908.
DOI: 10.1063/1.3597363
Google Scholar
[10]
V. Paul-Boncour, S. M. Filipek, R. Sato, R. Wierzbicki, G. André, F. Porcher, M. Reissner, G. Wiesinger, Journal of Solid State Chemistry 184 (2011)463–469.
DOI: 10.1016/j.jssc.2010.12.017
Google Scholar
[11]
M. T. Klepka, A. Wolska, K. Lawniczak-Jablonska, S. M. Filipek, R. Sato, V. Paul-Boncour, I. Marchuk, Radiation Physics and Chemistry 80 (2011) 1019–1025.
DOI: 10.1016/j.radphyschem.2011.03.016
Google Scholar
[12]
V. M. Goldschmidt, Skrifter Norske Videnskaps - Akad. Oslo. Mat - Naturv Kl. 1 (1926) 117.
Google Scholar
[13]
S. Takagi, K. Miwa, T. Ikeshoji, M. Matsuo, M. Kano, S. Orimo, To be publihed in Applied Physics Letters (2011).
Google Scholar
[14]
K. Yvon, G. Renaudin, Hydrodes: Solid State Transition Metal Complexes, in: R. B. King (Eds. ), Encyclopedia of Inorganic Chemistry, second ed., John Wiley & Sons, Ltd, Chichester, 2005, pp.1814-1846.
Google Scholar
[15]
S. Deledda, B. C. Hauback, Nanotechnology 20 (2009) 204010.
Google Scholar