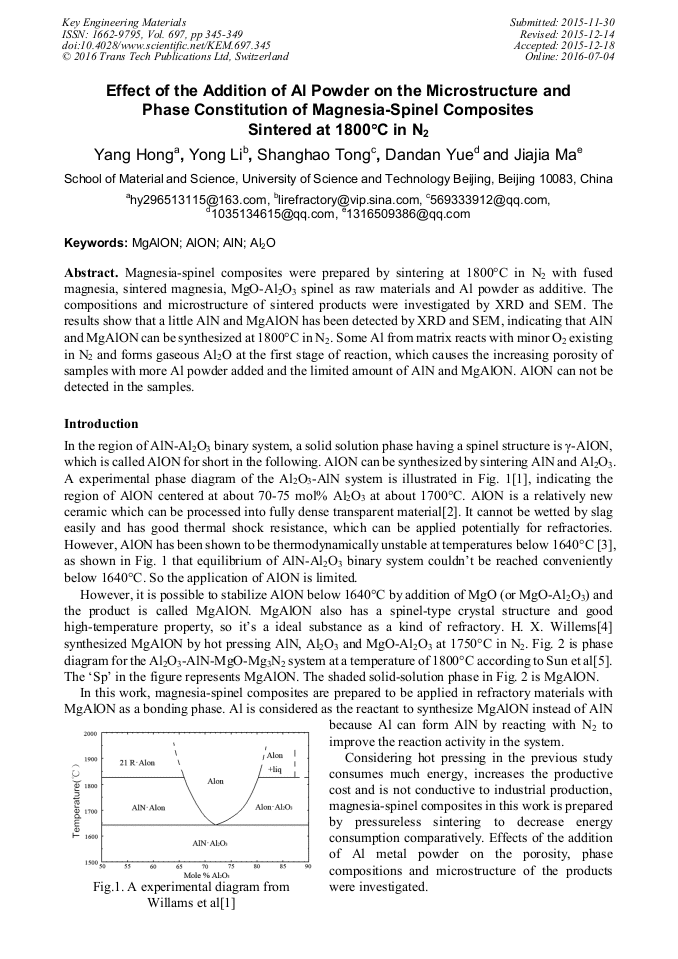
p.327
p.333
p.337
p.341
p.345
p.350
p.354
p.360
p.364
Effect of the Addition of Al Powder on the Microstructure and Phase Constitution of Magnesia-Spinel Composites Sintered at 1800°C in N2
Abstract:
Magnesia-spinel composites were prepared by sintering at 1800°C in N2 with fused magnesia, sintered magnesia, MgO-Al2O3 spinel as raw materials and Al powder as additive. The compositions and microstructure of sintered products were investigated by XRD and SEM. The results show that a little AlN and MgAlON has been detected by XRD and SEM, indicating that AlN and MgAlON can be synthesized at 1800°C in N2. Some Al from matrix reacts with minor O2 existing in N2 and forms gaseous Al2O at the first stage of reaction, which causes the increasing porosity of samples with more Al powder added and the limited amount of AlN and MgAlON. AlON can not be detected in the samples.
Info:
Periodical:
Pages:
345-349
DOI:
Citation:
Online since:
July 2016
Authors:
Price:
Сopyright:
© 2016 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: