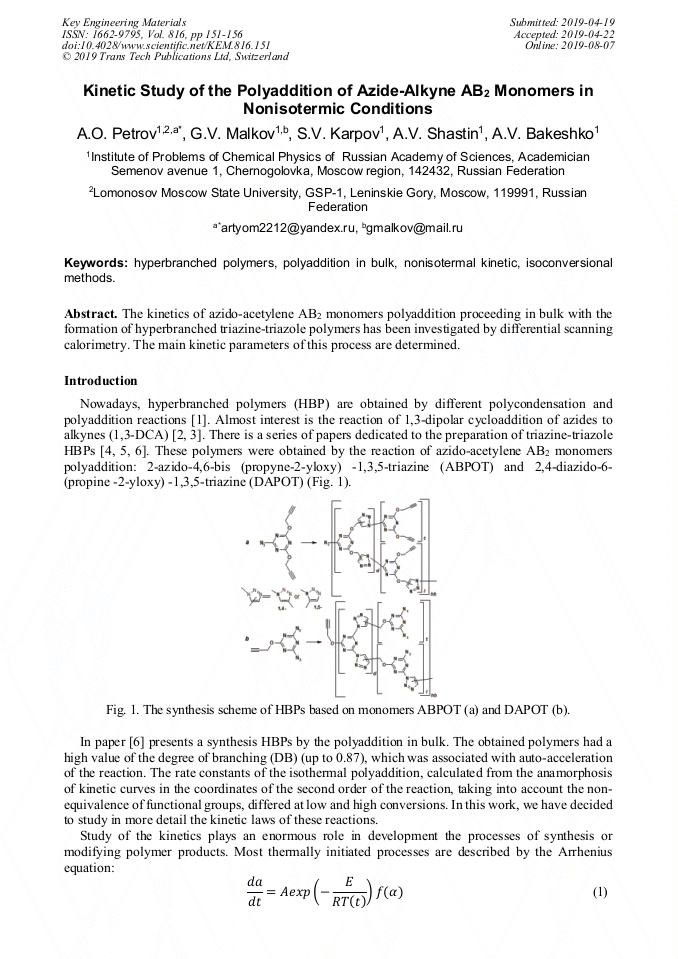
[1]
Y. Zheng et al, Hyperbranched polymers: advances from synthesis to applications, Chemical Society Reviews. 44 (2015) 4091-4130.
DOI: 10.1039/c4cs00528g
Google Scholar
[2]
R. Huisgen, 1, 3‐dipolar cycloadditions. Past and future, Angewandte Chemie International Edition. 2 (1963) 565-598.
DOI: 10.1002/anie.196305651
Google Scholar
[3]
V. O. Rodionov, V. V. Fokin, M. G. Finn, Mechanism of the Ligand‐Free Cu(I)‐Catalyzed Azide–Alkyne Cycloaddition Reaction, Angewandte Chemie. 117 (2005) 2250-2255.
DOI: 10.1002/ange.200461496
Google Scholar
[4]
G. V. Malkov et al., New polynitrogen hyperbranched polymers, Russian Chemical Bulletin. 60 (2011) 1940-1943.
Google Scholar
[5]
G. V. Malkov et al., New hyperbranched poly ([1, 2, 3]-triazole-[1, 3, 5]-triazines), Polymer Science Series B. 49 (2007) 301-304.
Google Scholar
[6]
G. V. Malkov et al., Hyperbranched Poly ([1, 2, 3]‐triazole‐[1, 3, 5]‐triazine) s: An Unusual High Degree of Branching as an Effect of a Polyaddition Kinetics, Macromolecular symposia. – Weinheim. 296 (2010) 107-111.
DOI: 10.1002/masy.201051016
Google Scholar
[7]
S. A. Vyazovkin, Time to search: finding the meaning of variable activation energy, Physical Chemistry Chemical Physics. 18 (2016) 18643-18656.
DOI: 10.1039/c6cp02491b
Google Scholar
[8]
A. O. Konuray, X. Fernández-Francos, X. Ramis, Analysis of the reaction mechanism of the thiol–epoxy addition initiated by nucleophilic tertiary amines, Polymer Chemistry. 8 (2017) 5934-5947.
DOI: 10.1039/c7py01263b
Google Scholar
[9]
S. A. Vyazovkin, N. Sbirrazzuoli, Mechanism and kinetics of epoxy− amine cure studied by differential scanning calorimetry, Macromolecules. 29 (1996) 1867-1873.
DOI: 10.1021/ma951162w
Google Scholar
[10]
J. M. Salla et al., Isoconversional kinetic analysis of a carboxyl terminated polyester resin crosslinked with triglycidyl isocyanurate (TGIC) used in powder coatings from experimental results obtained by DSC and TMDSC, Thermochimica acta. 388 (2002) 355-370.
DOI: 10.1016/s0040-6031(02)00036-9
Google Scholar
[11]
A. Tejado et al., Isoconversional kinetic analysis of novolac-type lignophenolic resins cure, Thermochimica Acta. 471(2008) 80-85.
DOI: 10.1016/j.tca.2008.03.005
Google Scholar
[12]
S. A. Vyazovkin et al., ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data, Thermochimica acta. 520 (2011) 1-19.
DOI: 10.1016/j.tca.2011.03.034
Google Scholar
[13]
S. A. Vyazovkin et al., ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations, Thermochimica Acta. 590 (2014) 1-23.
DOI: 10.1016/j.tca.2014.05.036
Google Scholar
[14]
H. L. Friedman, Kinetics of thermal degradation of char‐forming plastics from thermogravimetry. Application to a phenolic plastic, Journal of Polymer Science: Polymer Symposia. 6 (1964) 183-195.
DOI: 10.1002/polc.5070060121
Google Scholar
[15]
T. Akahira, T. Sunose, Method of determining activation deterioration constant of electrical insulating materials, Res Rep Chiba Inst Technol (Sci Technol). 16 (1971) 22-31.
Google Scholar
[16]
J. M. Criado, M. Gonzalez, The method of calculation of kinetic parameters as a possible cause of apparent compensation effects, Thermochimica Acta. 46 (1981) 201-207.
DOI: 10.1016/0040-6031(81)80244-4
Google Scholar