p.1135
p.1139
p.1143
p.1149
p.1153
p.1157
p.1163
p.1167
p.1171
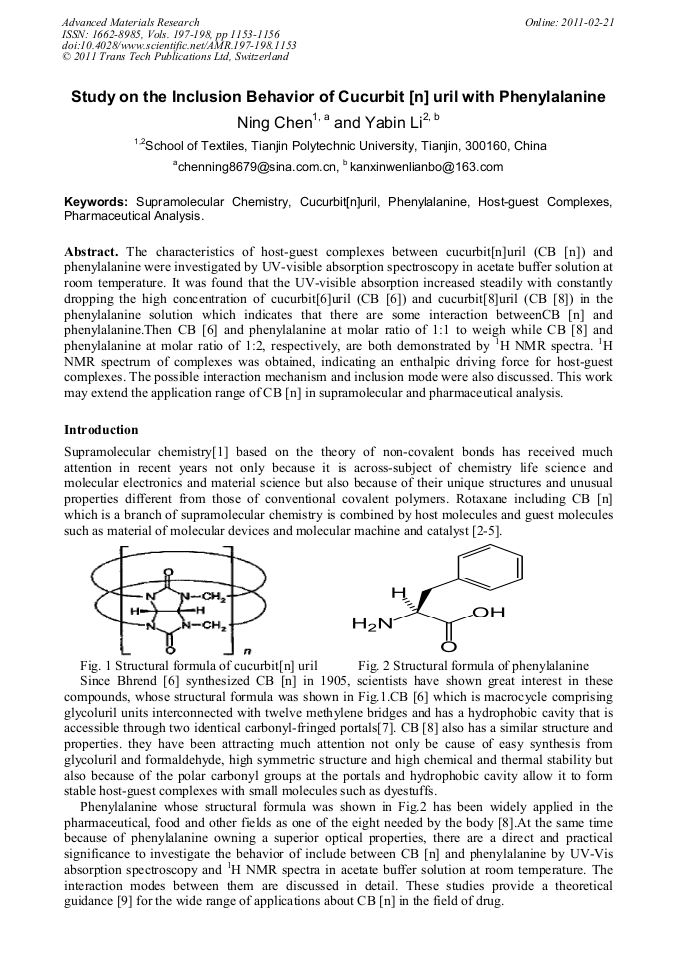
Study on the Inclusion Behavior of Cucurbit [n] uril with Phenylalanine
Abstract:
The characteristics of host-guest complexes between cucurbit[n]uril (CB [n]) and phenylalanine were investigated by UV-visible absorption spectroscopy in acetate buffer solution at room temperature. It was found that the UV-visible absorption increased steadily with constantly dropping the high concentration of cucurbit[6]uril (CB [6]) and cucurbit[8]uril (CB [8]) in the phenylalanine solution which indicates that there are some interaction betweenCB [n] and phenylalanine.Then CB [6] and phenylalanine at molar ratio of 1:1 to weigh while CB [8] and phenylalanine at molar ratio of 1:2, respectively, are both demonstrated by 1H NMR spectra. 1H NMR spectrum of complexes was obtained, indicating an enthalpic driving force for host-guest complexes. The possible interaction mechanism and inclusion mode were also discussed. This work may extend the application range of CB [n] in supramolecular and pharmaceutical analysis.
Info:
Periodical:
Pages:
1153-1156
Citation:
Online since:
February 2011
Price:
Сopyright:
© 2011 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: