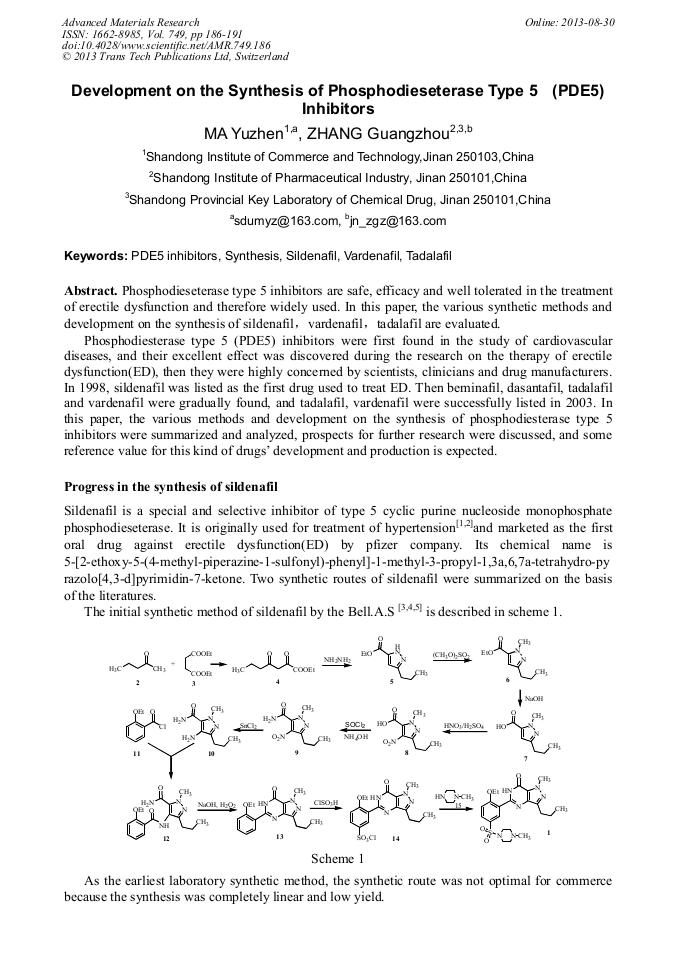
[1]
Kling, J. From hypertension to angina to Viagra[J]. Modern Drug Discovery, 1998, 1: 31-38.
Google Scholar
[2]
Campbell, S. F. Science, art and drug discovery: a personal perspective[J]. Clinical Science, 2000, 99, 255-260.
DOI: 10.1042/cs0990255
Google Scholar
[3]
Bell A.S. Brown,D. Terrett N.K. Pyrazolopy-rimidinones antianginal agents[P]. EP463756(1992).
Google Scholar
[4]
Bell A.S. Brown,D. Terrett N.K. Pyrazolopyrimidinone antianginal agents [P]. US5250534(1993).
Google Scholar
[5]
Terrett, N.K., Bell A.S. Brown,D. Ellis,P. Bioorg. Med. Chem. Lett. 1996, 6: 1819-1824.
Google Scholar
[6]
Dunn, P. J. Wood, A. S. Process for preparing sildenafil[P]. EP0812845 (1997).
Google Scholar
[7]
Liu Yuhui, Zhang Zhanbin. Method for preparing sildenafil [P]. CN01131151. 7(2001).
Google Scholar
[8]
Dunn P.J. Process for the preparation of pyrazolopyrimidinones [P]. WO 2001098284(2001).
Google Scholar
[9]
Xiong Zhenhu, Fei Xuening, Chang Kun. A New Synthesis of 1-Methyl-3-propylpyrazole-5-carboxlic Acid[J]. Chinese Journal of Synthetic Chemistry,2001,9(6):443-556.
Google Scholar
[10]
DAI Huifang, WANG Honghua, XIONG Fangjun, CHEN Fener. Graphical Synthetic Routes of Sildenafil [J]. Chinese Journal of Pharmaceuticals,2010,41(12):948-950.
Google Scholar
[11]
PETERJ D. Synthesis of commercial phosphodiesterase(V)inhibitors[J] . Organic Process Research & Development, 2005, 9(1): 88-97.
Google Scholar
[12]
Marc Nowakowski. Method for the production of 2-(2-ethoxyphenyl)-substituted imidazotriazinones[P]. US20040097505 (2004).
Google Scholar
[13]
Hatzimouratidis K, Hatzichristou D G. A comparative reviewof the options for treatment of erectile dysfunction: which treatment for whichpatient? [ J]. Drugs, 2005, 65(12) : 1621-1650.
DOI: 10.2165/00003495-200565120-00003
Google Scholar
[14]
Yao Shaobo, Cai Mingde, Lu Xiaoyi, Jiang Shende. Recent development on the synthesis of tadalafil [J]. Fine and Specialty Chemicals,2011,19(10):37-44.
Google Scholar
[15]
Dugan A. Tetracylic derivatives, process of preparation and use[P]. US5859006(1999).
Google Scholar
[16]
Dugan A, Grondin P, Ruault C, et al. The discovery of tadalafil: a novel and highly selective PDE5 inhibitor. 2, 2, 3, 6, 7, 12, 12-α-hexahydropyrazino[1', 2'-1, 6-pyrido-3, 4-b] inbole-1, 4- dione [J]. J Med. Chem., 2003, 46: 4533-4542.
DOI: 10.1021/jm0300577
Google Scholar
[17]
Deshpande P B,Boda B B,Surti S S,et al. Process for the preparing tadalafil and its intermediate[P]. US7223863(2006).
Google Scholar
[18]
Bhushan L B, Bhushan L V, Indulal P S. Process for the preparing tadalafil and its intermediate[P]. WO2005068464(2005).
Google Scholar
[19]
Revell J D, Srinivasan N, Ganesan A. Two concise synthesis of cialis via the N-acyliminium Pictet Spengler reaction[J]. Synlett, 2004, 1428-1430.
DOI: 10.1055/s-2004-825625
Google Scholar
[20]
Orme M W, Martinelli M J, Doecke C W, et al. Modified Pictet-Spengler reaction and products prepared therefrom[P]. US7550479(2009).
Google Scholar