p.108
p.114
p.130
p.136
p.141
p.150
p.160
p.167
p.173
Novel Magnesia Carbon Slide Gate Refractory Material for Corrosive Steel Application
Abstract:
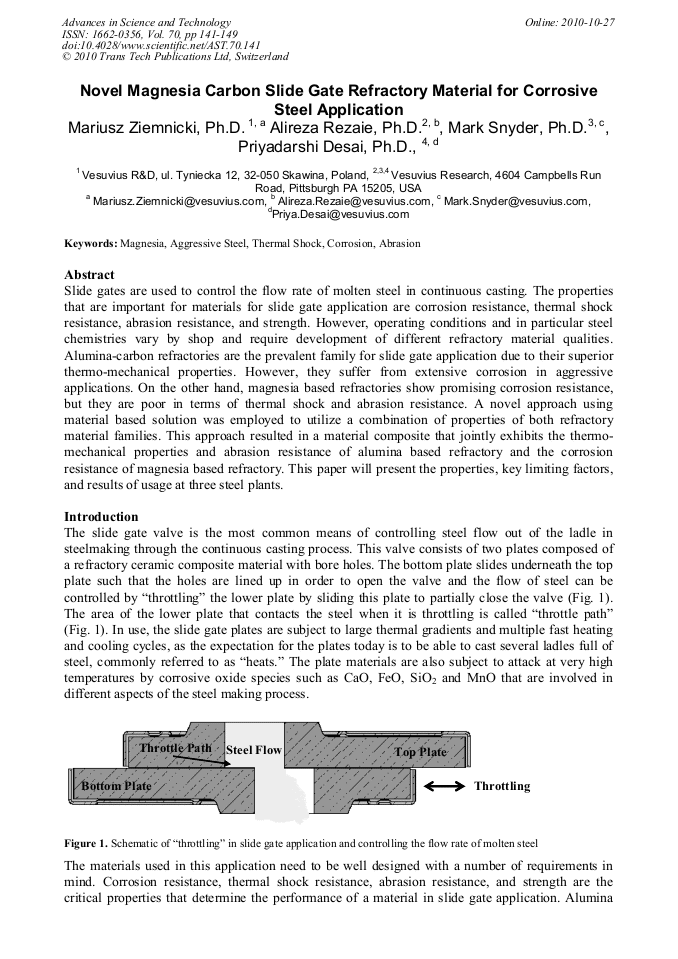
Slide gates are used to control the flow rate of molten steel in continuous casting. The properties that are important for materials for slide gate application are corrosion resistance, thermal shock resistance, abrasion resistance, and strength. However, operating conditions and in particular steel chemistries vary by shop and require development of different refractory material qualities. Alumina-carbon refractories are the prevalent family for slide gate application due to their superior thermo-mechanical properties. However, they suffer from extensive corrosion in aggressive applications. On the other hand, magnesia based refractories show promising corrosion resistance, but they are poor in terms of thermal shock and abrasion resistance. A novel approach using material based solution was employed to utilize a combination of properties of both refractory material families. This approach resulted in a material composite that jointly exhibits the thermomechanical properties and abrasion resistance of alumina based refractory and the corrosion resistance of magnesia based refractory. This paper will present the properties, key limiting factors, and results of usage at three steel plants.
Info:
Periodical:
Pages:
141-149
DOI:
Citation:
Online since:
October 2010
Authors:
Keywords:
Price:
Сopyright:
© 2010 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: