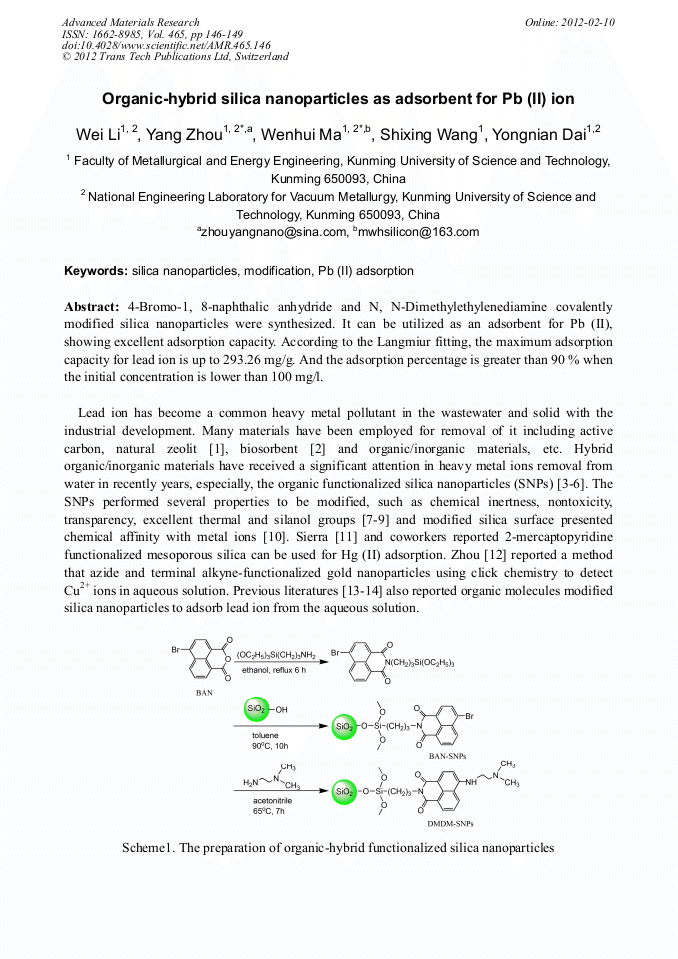
[1]
J. Perić, M. TRgo, N. V. Medvidović, Water Res. 38 (2004) 1893–1899.
Google Scholar
[2]
Rm. Gong, Y. Ding, Hj. Liu, Qy. Chen, Zl. Liu, Chemosphere 58 (2005) 125–130.
Google Scholar
[3]
N. L. D. Filho, D. R. do Carmo, A. H. Rosa, Electrochim. Acta 52 (2006) 965-972.
Google Scholar
[4]
L. N. H. Arakaki, A. G. da Fonseca, E. C. da Silva Filho, A. P de. M. Alves, K. S. de Sousa, A. L. P. Silva, Thermochim. Acta 450 (2006) 12-15.
DOI: 10.1016/j.tca.2006.06.012
Google Scholar
[5]
E. F .C. Alcântara, E. A. Faria, D. V. Rodrigues, S. M. Evangelista, E. DeOliveira, L. F. Zara, D. Rabelo, A. G. S. Prado, J. Colloid Interface Sci. 311 (2007) 1-7.
DOI: 10.1016/j.jcis.2007.02.075
Google Scholar
[6]
J. Aguado, J. M. Arsuaga, A. Arencibia, M. Lindo, V. Gascón, J. Hazard. Mater. 163 (2009) 213–221.
Google Scholar
[7]
B. Zhao, W. J. Brittain, Prog. Polym. Sci. 25 (2000) 677–710.
Google Scholar
[8]
D. Hua, J. Tang, J. L. Jiang, Z. Q. Gu, L. L. Dai, X. L. Zhu, Mater. Chem. Phys. 114 (2009) 402–406.
Google Scholar
[9]
J. Yang, S. J Ding, M. Radosz,Y. Q. Shen, Macromolecules 37 (2004) 1728-1734.
Google Scholar
[10]
A. G. S. Prado, B. S. Miranda, G. V. M. Jacinto, Surf. Sci. 542 (2002) 276-282.
Google Scholar
[11]
D. P. Quintanilla, I. del Hierro, M. Fajardo, I. Sierra, Microporous Mesoporous Mat. 89 (2006) 58-68.
DOI: 10.1016/j.micromeso.2005.10.012
Google Scholar
[12]
Y. Zhou, S. Wang, K. Zhang, X. Jiang, Angew. Chem. Int. Ed. 47 (2008) 7454-7456.
Google Scholar
[13]
X. Liang, Ym. Xu, Gh. Sun, L. Wang, Y. Sun, X. Qin, Physicochem. Eng. Aspects 349 (2009) 61-68.
Google Scholar
[14]
N. Chiron, R. Guilet, E. Deydier, Water Res. 37 (2003) 3079–3086.
Google Scholar
[15]
D. Zhang, Xm. Wang, Za. Qiao, Dh. Tang, Yl. Liu, Qs. Huo, J. Phys. Chem. C 114 (2010) 12505–12510.
Google Scholar
[16]
C. Niu, Gm. Zeng, Lx. Chen, Gl. Shen, Rq. Y, Analyst 129 (2004) 20-24.
Google Scholar
[17]
Y. Zhao, Hy. Shen, Q. Li, Qh. Xia, Acta Chim. Sinica 67 (2009) 1509-1514.
Google Scholar