p.2570
p.2574
p.2581
p.2586
p.2594
p.2598
p.2610
p.2615
p.2619
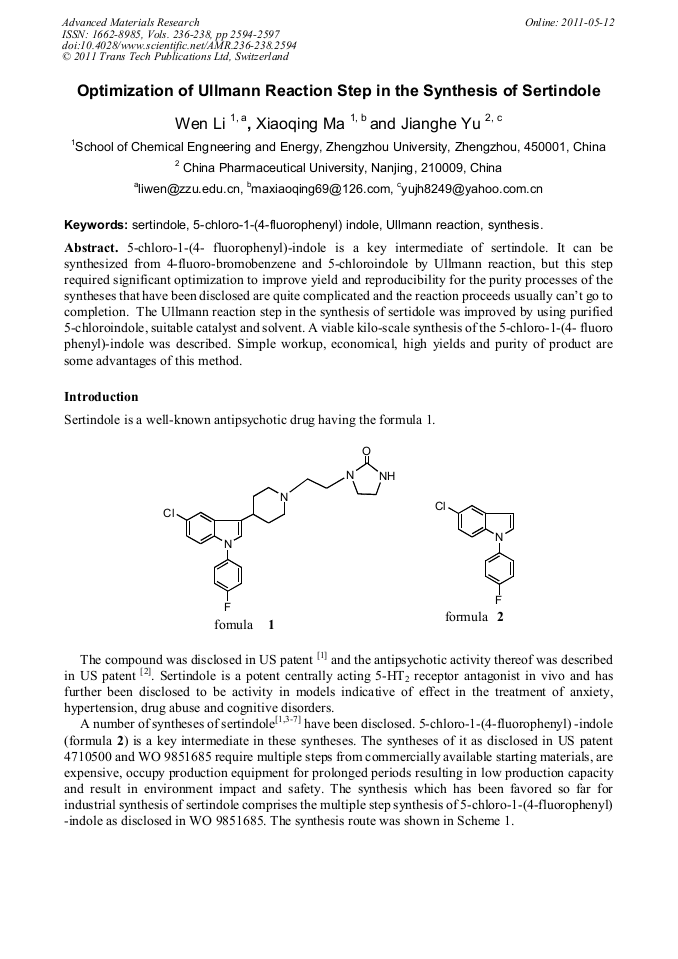
Optimization of Ullmann Reaction Step in the Synthesis of Sertindole
Abstract:
5-chloro-1-(4- fluorophenyl)-indole is a key intermediate of sertindole. It can be synthesized from 4-fluoro-bromobenzene and 5-chloroindole by Ullmann reaction, but this step required significant optimization to improve yield and reproducibility for the purity processes of the syntheses that have been disclosed are quite complicated and the reaction proceeds usually can’t go to completion. The Ullmann reaction step in the synthesis of sertidole was improved by using purified 5-chloroindole, suitable catalyst and solvent. A viable kilo-scale synthesis of the 5-chloro-1-(4- fluoro phenyl)-indole was described. Simple workup, economical, high yields and purity of product are some advantages of this method.
Info:
Periodical:
Pages:
2594-2597
Citation:
Online since:
May 2011
Authors:
Price:
Сopyright:
© 2011 Trans Tech Publications Ltd. All Rights Reserved
Share:
Citation: