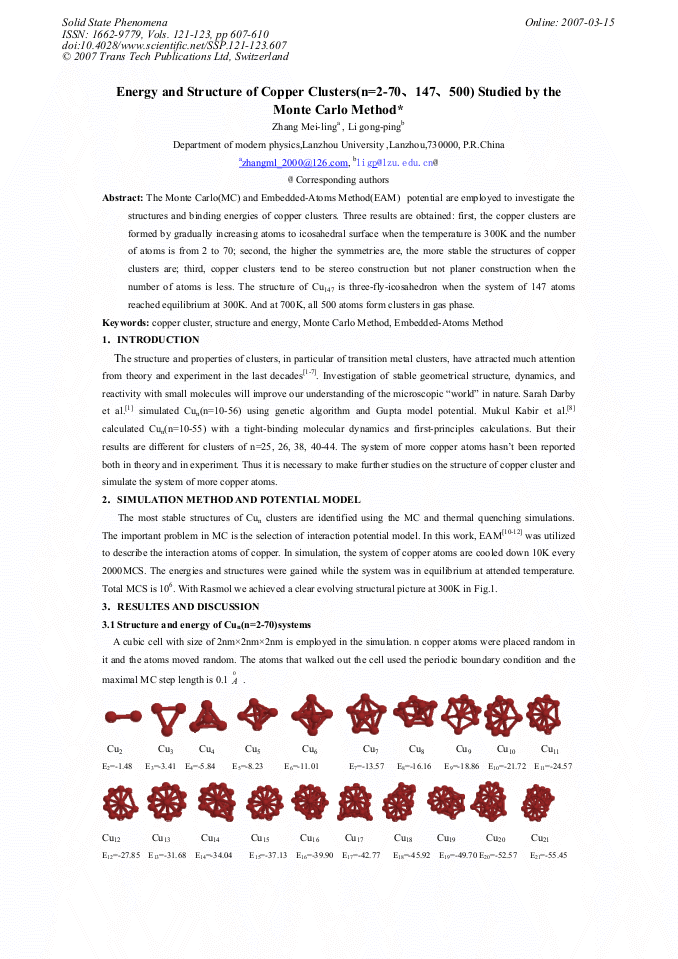
[1]
Sarah Darby, Thmas V. Mortimer-Jones, Rog L. Johnston, et al. Theoretical study of Cu-Au nanoalloy clusters using a genetic algorithm[J]. J. Chem. Phys., 2002, 116: 1536.
DOI: 10.1063/1.1429658
Google Scholar
[2]
Süleyman Özcelik and Ziya B. Güvenc, Structures and melting of Cun(n=13, 14, 19, 55, 56) clusters, Surf. Sci., 2003, 532-535: 312.
DOI: 10.1016/s0039-6028(03)00432-1
Google Scholar
[3]
E.K. Parks,L. Zhu,J. Ho, et al. The structure of small nickel clusters. I. Ni3-Ni15, J. Chem. Phys. 1994, 100: 7206.
Google Scholar
[4]
M.S. Stave A.E. DePristo, The structure of NiN and PdN clusters: 4 <= align="bottom"> N <=" align="bottom, >23, J. Chem. Phys., 1992, 97: 3386.
Google Scholar
[5]
S. Krüger,S. Vent,F. NÖrtemann,M. Staufer, et al. The average bond length in Pd clusters Pdn, nƒ4 - 309: A density-functional case study on the scaling of cluster properties, J. Chem. Phys., 2001, 115(5): (2082).
DOI: 10.1063/1.1383985
Google Scholar
[6]
C. Rey L.J. Garcia-Rodeja, et al. Molecular-dynamics study of the binding energy and melting of transition-metal clusters, Phys. Rev. B, 1993, 48: 8253.
DOI: 10.1103/physrevb.48.8253
Google Scholar
[7]
J. Garcia-Rodeja,C. Rey, L.J. Garcia-Rodeja, et al. Molecular-dynamics study of the structures, binding energies, and melting of clusters of fcc transition and noble metals using the Voter and Chen version of the embedded-atom model, Phys. Rev. B, 1994, 49: 8495.
DOI: 10.1103/physrevb.49.8495
Google Scholar
[8]
Mukul Kabir and Abhijit Mookerjee, Structure and stability of copper clusters: A tight-binding molecular dynamics study[J]. Phys. Rev., 2004, A69: 043203.
DOI: 10.1103/physreva.69.043203
Google Scholar
[9]
Guo X Y, Formation and Growth Mechanism of Pdn Clusters Studied by the Monte Carlo Method, [J], in chinese, J. phys. chem, 2003, 19(2): 174.
Google Scholar
[10]
Liu L, Yang W D, Zi J, et al. Structure and Cohesive Energy of Cu Clusters(Ⅰ)[J], in chinese, J. chem. phys, 1993, 34: 314.
Google Scholar
[11]
Murray S. Daw. , M.I. Baskes, Semiempirical, Quantum Mechanical Calculation of Hydrogen Embrittlement in Metals Phys. Rev. Lett., 1983, 50: 128.
DOI: 10.1103/physrevlett.50.1285
Google Scholar
[12]
Murray S. Daw. , M.I. Baskes, Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals, Phys. Rev., 1984, B29: 6443.
DOI: 10.1103/physrevb.29.6443
Google Scholar
[13]
N.T. Wilson, Ph.D. thesis, University of Birmingham, (2000).
Google Scholar